The following sections will help explain the necessary information required to input into the drug database fields.
Generic Name #
Choose the drug’s generic name from the drop-down box.
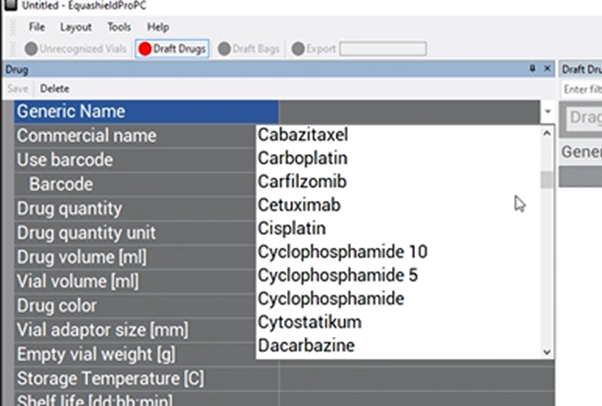
If the drug is not listed on the list, perform the following steps to add it to the list:
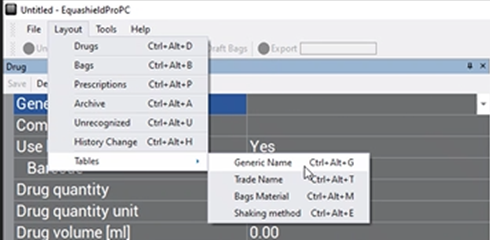
Adding a Drug Name to the Drop-down Box List #
- Click Layout
- Click Tables
- Click Generic Name or Trade Name (depending on your needs)
4. A new screen will appear.
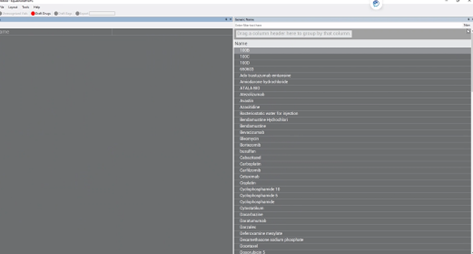
5. On the left side, click on “Name”.
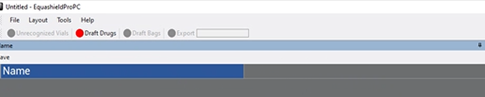

6. Type the name in the free text box next to “Name”.
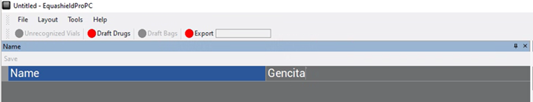
7. Press “Save” (above “Name”).
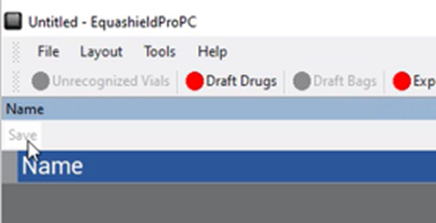
8. The name will now be on the right side and in your drop-down box. Go back to ‘Draft drugs to continue.

Commercial Name (Trade Name) #
Choose the drug’s commercial name from the drop-down box.
Note that the commercial name is also called “Trade name”.
If the drug is not listed in the drop-down box, follow the previous steps (under the “Adding a Drug Name to the Drop-down Box List” heading.)
It is very important to make the commercial name match the name that is used in the facility’s prescribing software.
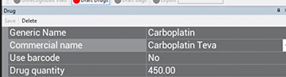
*The generic name and commercial name cannot be the same. If it is the same, add the manufacturer’s name to the commercial name. Ex: Carboplatin Teva
Use Barcode #
Always choose “No” from the drop-down box to choose.
This field is for EQUASHIELD® purposes only.
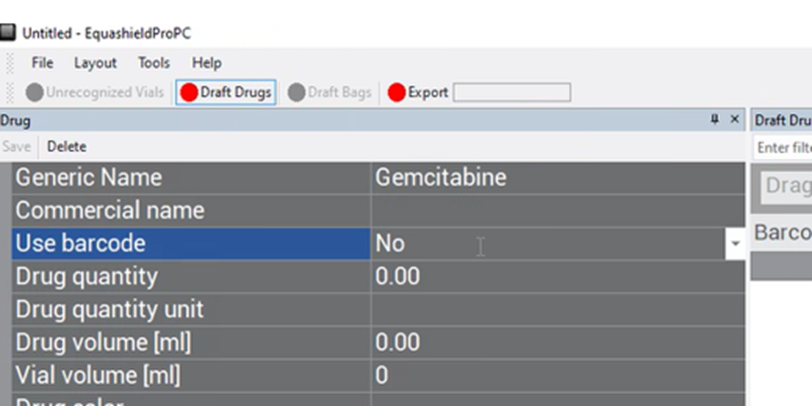
Drug Quantity #
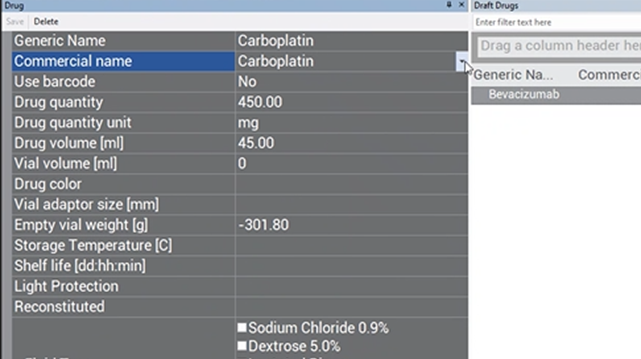
Enter in the free-text box the mass of the drug in relation to its volume (drug concentration that can be found on the vial label).
Ex: In the image of CARBOplatin below, free text “450”.
(for 450 mg in relation to 45ml)

When there are multiple drug concentrations listed on the vial, choose the option that represents the whole vial.
Ex: In the image of CARBOplatin above, there are 2 drug concentrations listed:
- 450 mg/ 45 ml
- 10 mg/ ml
Use the first concentration of 450 mg/ 45 ml as the reference since there are 45 ml of drug in the vial, and this concentration represents the whole vial. Enter “450” in the free-text box.
Drug Quantity Unit #
Choose the unit of measurement in the drop-down box for ‘Drug Quantity’ (previous field).
There are only 3 options: IU, mcg, and mg
If the unit of measurement is not in the drop-down box, do the conversion to make it match one of the units available.
If a conversion is made, ensure that the ‘Drug Quantity’ field reflects the correct number based on the units chosen.
Ex: If the Drug Quantity is 2g. Convert to 2000mg.
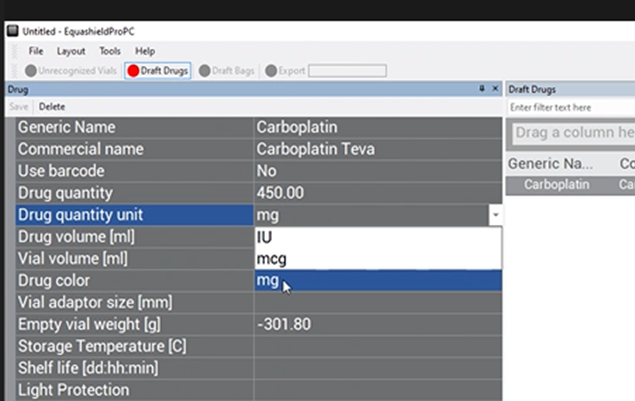
Drug Volume (ml) #
Enter the volume of the drug in ml in the free-text box in relation to its mass (concentration of drug that can be found on vial label).
Ex: In the image of CARBOplatin below, free text “45”.
(for 45 ml in relation to 450 mg)

When there are multiple drug concentrations listed on the vial, choose the option that represents the whole vial.
Ex: In the image of CARBOplatin above, there are 2 drug concentrations listed:
- 450 mg/ 45 ml
- 10 mg/ ml
Use the first concentration of 450 mg/ 45 ml as the reference since there are 45 ml of drug in the vial, and this concentration represents the whole vial. Enter “45” in the free-text box.
Vial Volume (ml) #
Enter the total volume of drug in the vial in ml in the free-text box. Often, this will be the same value as “Drug Volume (ml)”.
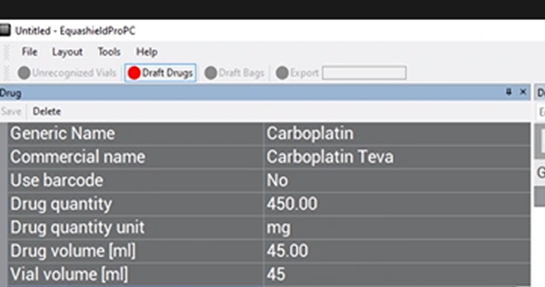
Ex: In the image of CARBOplatin below, free text “45”.
(for 45ml total volume in vial)

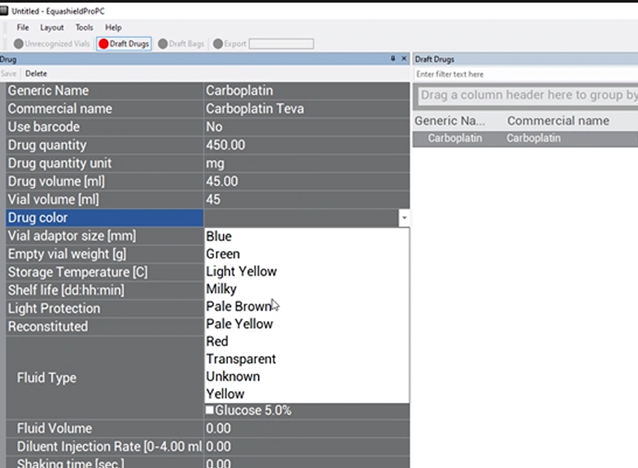
Drug Color #
Choose the color of the drug (NOT the color of the vial) from the drop-down box.
Vial Adaptor Size (mm) #
Choose what size vial adaptor is needed for this vial from the drop-down box.
The number in the drop-down box refers to the diameter in mm of the vial neck.
*Choose 13 if using VA-13/2 or VA-13C
*Choose 20 if using VA-20/2 or VA-20C
*Choose 28 if using VA-28/2
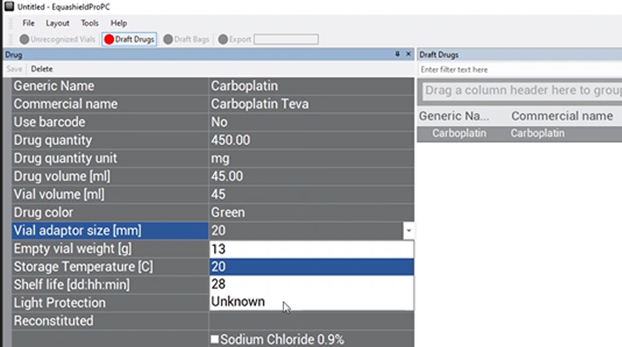

Empty Vial Weight (g) #
This field refers to the weight of the empty vial with an affixed vial adaptor.
*If it is a drug that requires reconstitution, then weigh the vial + vial adaptor + lyophilized powder during the image processing step (that has not been reconstituted).
***Do not enter anything in this field.***
The weight should be automatically entered based on the weight that was determined during the image processing step.
Storage Temperature (c) #
Choose from the two options in the drop-down box the temperature conditions needed for storing this drug.
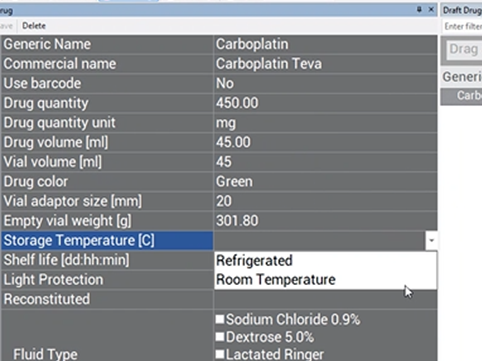
If the drug is temperature-sensitive, an icon will appear on the operator screen:

The EQUASHIELD® Pro will remove the vial from storage after compounding the prescription with a temperature-sensitive drug. However, future software versions will allow the Supervisor an option to enter the amount of time the drug can remain in the EQUASHIELD® Pro.
Shelf Life (dd:hh:mm) #
Enter in the free-text box the (days:hours:min) that the drug is chemically stable from the moment it is accessed (vial adaptor applied).
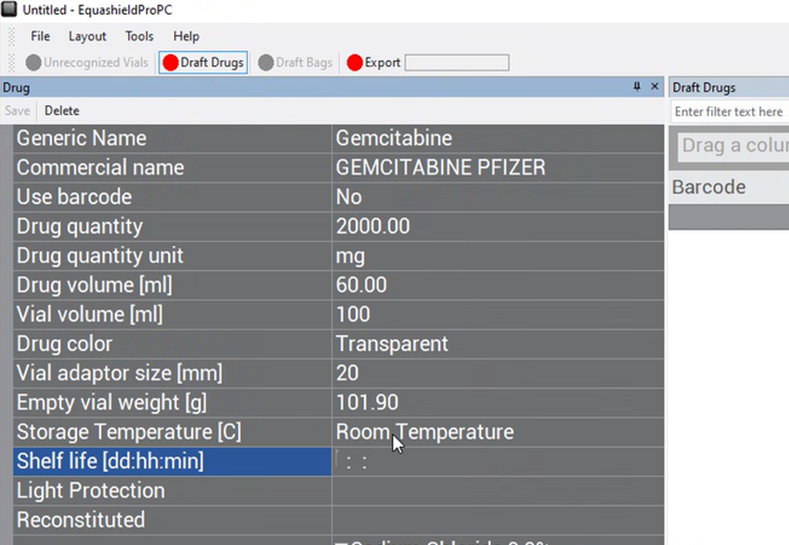
Light Protection #
Choose from the drop-down box the “Yes” or “No” options to indicate if the drug is light-sensitive.
If the drug is light-sensitive, an icon will appear on the operator screen:

The EQUASHIELD® Pro will remove the vial from storage after compounding the prescription with a light- sensitive drug. However, future software versions will allow the Supervisor an option to enter the amount of time the drug can remain in the EQUASHIELD® Pro.
Reconstituted #
Choose from the drop-down box the “Yes” or “No” options to indicate if the drug requires reconstitution.
The fields following fields indicated in the image below only refer to drugs that require reconstitution (and should only be filled in if the drug requires reconstitution).
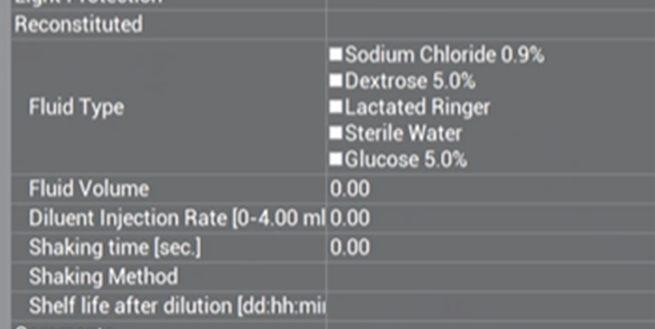
Reconstitution Overview #
The EQUASHIELD® Pro is able to perform reconstitution.
The Supervisor must enter all the parameters of the reconstitution process in the drug database prior to use. This is done in the Supervisor Application in the ‘drug database’.
It can be a learning curve on how to translate the manual process to the automated process. Don’t worry! The settings can always be modified.
The EQUASHIELD® Pro will only perform shaking after injecting the diluent fluid. It cannot re-shake something that was previously reconstituted. It cannot shake something on command.
Injecting and reconstitution parameters should be configured according to drug manufacturer recommendations and facility protocol.
Diluent fluid can ONLY be in IV bag form, and not from a semi-rigid bottle or vial (with the exception of customized machine).

Fluid Type (for reconstitution only) #
Check the boxes of the types of diluents that the drug can be reconstituted with. Check as many boxes as appropriate for the specific drug.
Fluid Volume (for reconstitution only) #
Type the volume of diluent in ml that should be used for reconstitution.
Diluent Injection Rate (for reconstitution only) #
Free-text the rate of injection of the diluent in ml/sec.
Options of 0ml-4ml ml/sec, with 4 ml/sec as the fastest option.
It may be a learning curve to understand the injection speed. This field can always be modified if needed.
Shaking Time (sec) (for reconstitution only) #
Free-text how long the vial should be in the ‘shaking process’ during reconstitution (includes shakes, inversions, etc.) in seconds.
There is no minimum or maximum parameter
Enter the time required for shaking according to manufacturer recommendations or facility protocol.
Shaking Method (for reconstitution only) #
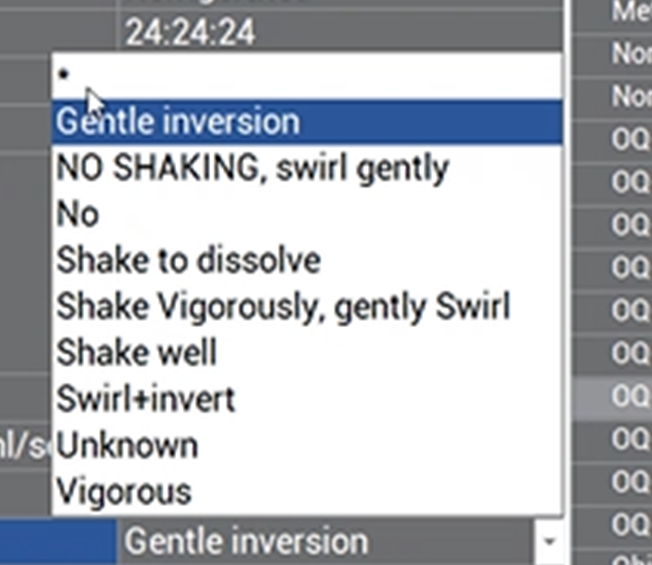
Enter the shaking method needed to reconstitute the drug according to manufacturer recommendations or facility protocol.
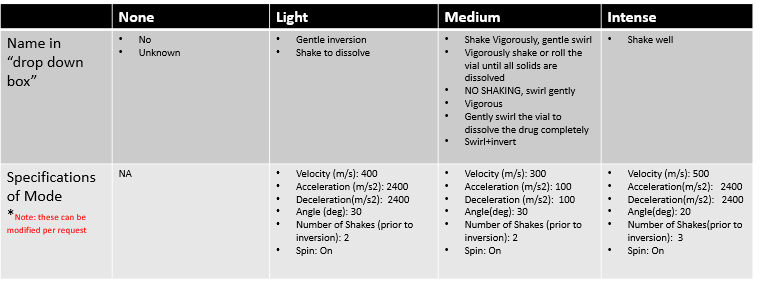
Shaking method: There are essentially only 4 shaking settings:
1.No shaking
2. Light shaking
3. Medium shaking
4. Intense shaking
It is important to understand the EQUASHIELD® terminology in order to determine the correct shaking method.
Terminology:
| Term | Action in Equashield® Pro |
| Shake | •Moves the vial side to side, typically 30 degrees side to side, •One movement Left + one movement right = 1 shake |
| Inversion/Spin | •Spins vial upside-down, 360-degree rotation •Typically, there is a designated number of shakes before inversion. EX: Shake, shake, shake, invert. Shake, shake, shake, invert. |
| Swirl | *There is no swirl capability. When it is recommended to swirl a vial, the action is replaced with inversion. |
Q:
If there are only 4 shaking options, then why are there so many options in the drop box?
A:
Manufacturers have various terminology for a similar action.
To make it easier for the operator, EQUASHIELD® took common action words from the manufacturer and translated them into automated shaking parameters.
The 4 shaking methods clarified:
Shelf Life After Dilution (dd:hh:mm) (for reconstitution only) #
Free-text the (days:hours:min) that the drug is chemically stable from the moment it has been reconstituted.
Comments #
Free-text any comments that may be relevant for the software team.

Sensitive Drugs #
Choose from the drop-down box “Yes” or “No” options to answer if the drug is sensitive (to other things that are not light or temperature sensitive).
If the drug is sensitive, an icon will appear on the operator screen:

The EQUASHIELD® Pro will remove the vial from storage after compounding the prescription with a sensitive drug. However, future software versions will allow the Supervisor an option to enter the amount of time the drug can remain in the EQUASHIELD® Pro.
EQ Vial ID #
Do not enter anything in this field.
*For EQUASHIELD® purposes only*

