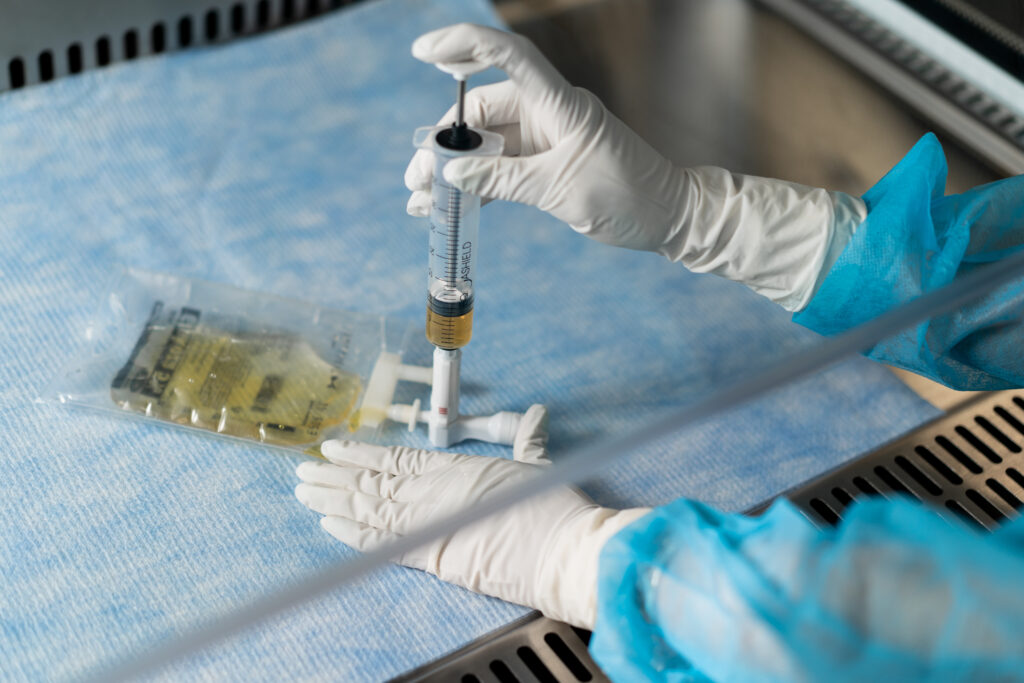
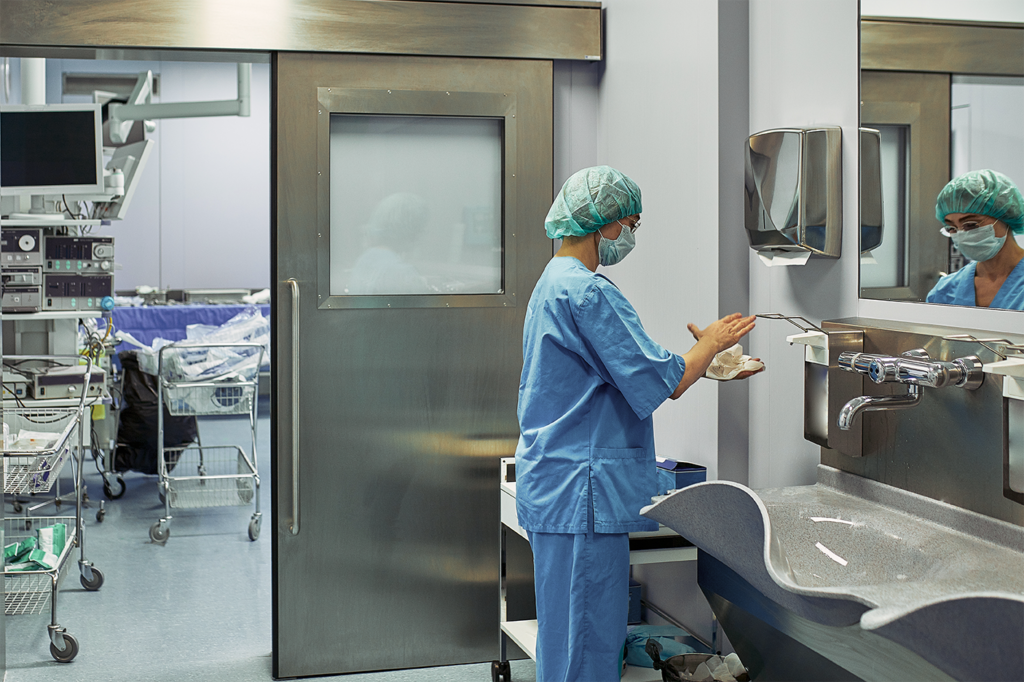
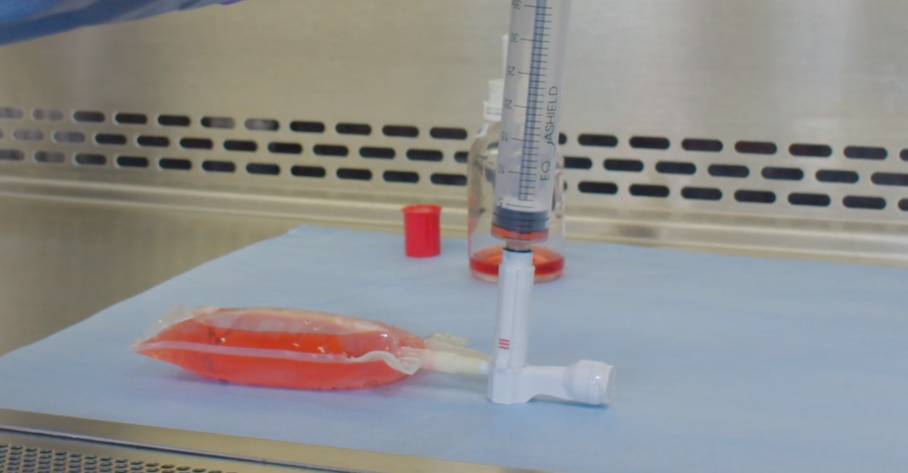
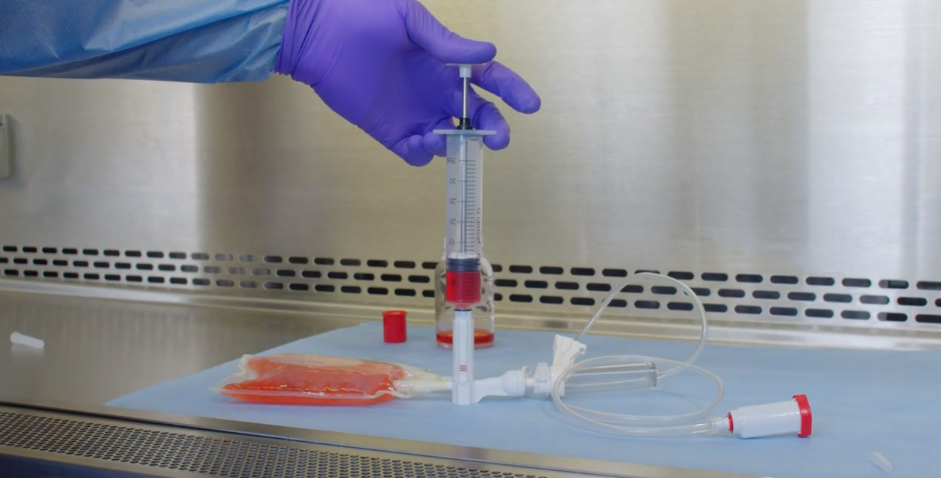
EQUASHIELD® has committed itself to protect healthcare workers from the risks associated with exposure to hazardous drugs and vapors.
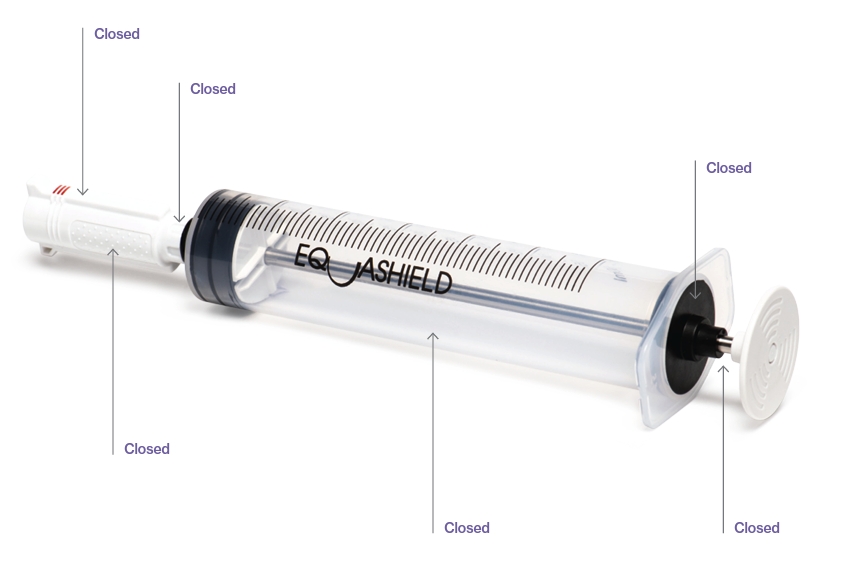
To provide advanced, automated, and innovative solutions to protect healthcare workers and facilities from the risks associated with hazardous drug exposure and contamination.
The EQ Academy page is offering advanced insights into the safe handling of hazardous drugs. Featuring an array of engaging blogs, how to videos, tutorials, podcasts and more.