Safeguarding Cell / Gene Therapy Manufacturing

CellSHIELD is an IP-protected fully closed device ensuring sterility throughout the cell / gene therapy manufacturing process. The product empowers biopharma companies and CDMOs alike to safely and efficiently scale-up manufacturing operations – often eliminating the need for a biosafety cabinet.
/ 01
/ 02
/ 03
/ 04
Years of Experience
Countries
Employees
Global Customers
Prevents release and ingress of extraneous biological agents and microbes.
No cross-contamination risk.
To vapors or disinfectants. Compatible with cleanroom disinfectants via liquids or vhp.
Clinically-validated.
No wastage during sampling… and every single dose is crucial.
Device suited for sampling mammalian cells & cell transfer without impacting cell viability.
High assurance of zero defects at point-of-use.
Quality Control
Therapy Manufacturing
Input Manufacturing
Discovery & Development
CellSHIELD™ offers a user-friendly and intuitive experience, requiring minimal training. This streamlines the onboarding process for newly-hired employees, allowing them to quickly adapt and contribute to the workforce.
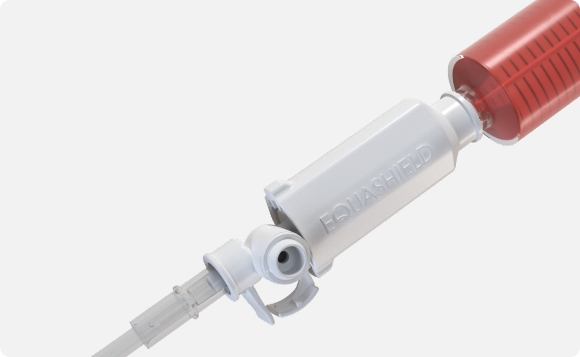
Fully encapsulated structure prevents syringe-plunger detachment and contamination; enables the device’s pressure equalisation system.

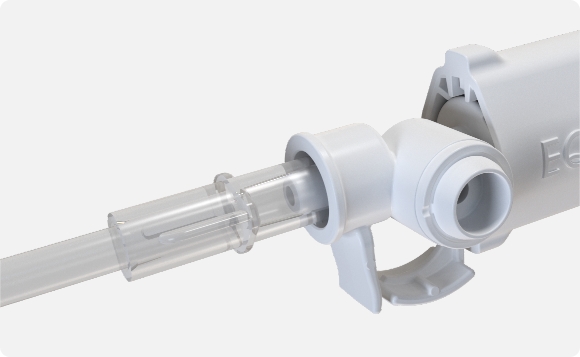
Air released to make way for the cell mixture flows through a needle in the syringe center and ultimately passes through a hydrophobic filter.

When the media is ultimately released from CellSHIELD™, incoming air passes through the filter to maintain sterility; the filter also allows EtO gas to pass through originally, before any product usage.