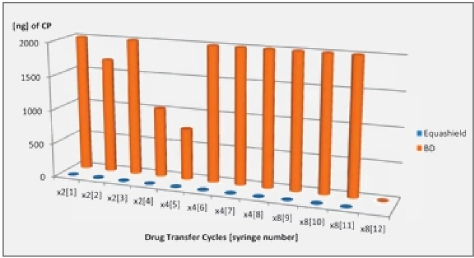
1 Background Information and Rationale
1.1 Background
Ideally, once meticulously designed, formed, and adequately used, a closed system transfer device (CSTD) should provide a protective shield from any hazardous exposures to healthcare workers during the compounding and administration of hazardous drugs (HDs). However, all CSTDs utilize a syringe, which may have a detrimental effect on the characterization of the system defined as a CSTD. An exception is a dedicated closed syringe unit that provides a closed system of its own. A regular open barrel syringe can potentially contaminate healthcare workers to various degrees depending on the drug used and its volatility, concentration, viscosity, and affinity to the syringe surface.1,2
The standard use of any HD requires filling a syringe with the drug. During the process of withdrawing the HD to the syringe, the HD comes into direct contact with the inner wall of the syringe. Hence, the syringe inner surface is directly exposed to the drug for a period of time. This may create a reaction, allowing the HD to stick to the syringe surface either by chemical affinity or by cohesive-adhesive forces of the HD. After the drug is transferred, the inner surface remains fully exposed to the environment, and potentially perilous contamination with the hazardous drug may occur by 2 possible routes: (1) by evaporation of the HD to the ambiance of the room or (2) by direct contact of the syringe plunger with the inner wall of the syringe. The latter type of contamination could be transferred via gloves of the technicians to other surfaces, resulting in spread of contamination in the working environment. Obviously, this should be prevented as much as possible and should not occur while a CSTD is used.
Different contamination results may be observed with individual HDs due to the unique physical and chemical properties of each drug.
1.2 Findings From Studies
Studies using CSTDs have shown a significant reduction in surface contamination levels, although detectable levels of hazardous substances were observed, suggesting that some systems are not entirely safe, and that healthcare workers remain at risk of exposure.3,4 A study using a surface monitoring technique further explored environmental contamination when it specifically examined the possibility of syringe plunger contamination during routine drug preparation at hospital pharmacies. Contamination by cyclophosphamide was confirmed, quantified, and localized on a standard syringe plunger.1 Results from additional studies confirmed these results with cyclophosphamide used in a CSTD that utilizes a standard syringe and revealed that drug residuals on the syringe plunger contaminate both gloves and the work environment.2,5
1.3 Study Objectives
The purpose of the study is (1) to establish evidence for HD contamination of the inner walls of regular syringes exposed to the environment and (2) to compare the intensity of contamination between commonly used HDs.
2 Protocol Overview
Three common HDs shall be evaluated in real-world conditions of use for contamination levels upon exposure to environmental surfaces of regular open barrel 50 mL syringes. A total of 50 mLs of drug will be transferred from vial to IV bag using a regular syringe and CSTD. The drugs of interest are cyclophosphamide, ifosfamide, and 5-fluorouracil. The CSTD PhaSeal will be used during each of the preparations.
ChemoGLO HD wipe kits are used for sampling the tested syringes.
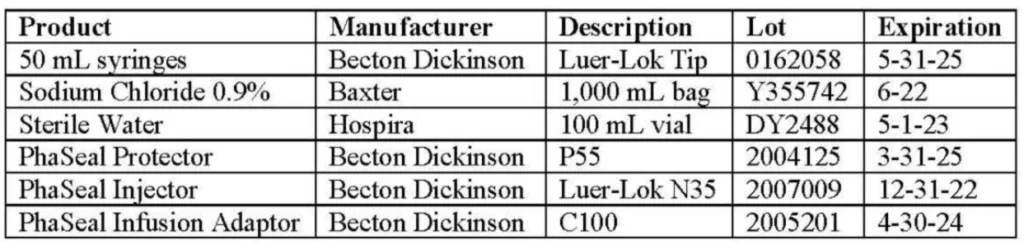
3 Description of Supplies
This study used the following materials.
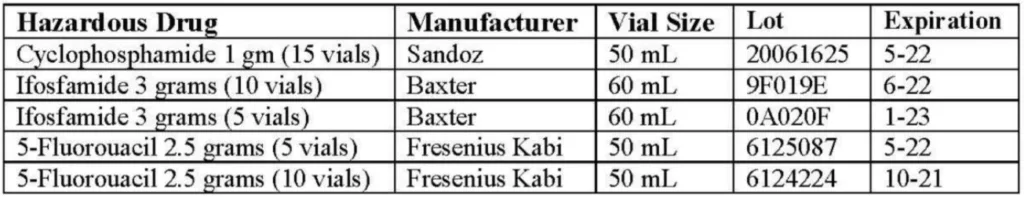
4 Selection of Drugs and Dosages
This study utilized 3 hazardous drugs to assess contamination.
5 Data Collection
Data was recorded on the ChemoGLO Site Map Form.
The completed ChemoGLO Site Map Form is included with the wipe samples that are sent to the lab.
6 Testing Conditions
Hospital pharmacy setting for handling and preparation of hazardous drugs (eg, cleanroom, BSC, PPE).
7 Test Procedure
- Remove the flip-cap from the drug vial and attach the CSTD vial adapter. Perform this and all next steps in accordance with the CSTD manufacturer’s IFU.
- Attach the CSTD bag adapter to the IV bag.
- Attach the syringe to the CSTD connector.
- Cyclophosphamide (CP) and ifosfamide (IF) require reconstitution; therefore, follow the instructions provided in the respective package insert and reconstitute the drugs as instructed.
- Connect the syringe to the vial.
- Invert the vial and draw 50 mL of drug.
- Disconnect the syringe from the vial and connect the syringe to the IV bag (at all stages using the CSTD).
- Inject the entire drug dose into the IV bag and disconnect.
- Using an adequate cutter tool, cut out a quarter from the plunger barrel knob. This step will enable controlled and easy access to the syringe barrel without interference with the tested syringe. This allows for wiping the exposed inner wall of the syringe.
- Pre-wet the ChemoGLO wipe in accordance with its IFU and insert the wipe into the space between the plunger and the barrel.
- Using a wooden rod. insert the wipe deep into the rear opening of the syringe. A single-use wooden rod is used to move the ChemoGLO wipe up and down the barrel of the syringe in the exposed syringe barrel (one quarter of the entire barrel).
- Push the wipe all the way down to the bottom of the syringe in 1 of the 4 spaces, thereby wiping the exposed inner wall of the syringe.
- The syringe plunger rod is rotated 90 degrees and the process is repeated. This occurs a total of 4 time to ensure that the entire syringe barrel is wiped. The wipe is removed.
- Pack and label the wipe in accordance with instructions provided in the sampling kit. The second ChemoGLO wipe is repeated on the syringe.
- Repeat the testing procedure a total of 15 times with the same drug (15×1=15 replicates).
- Repeat the previous process with each of the 3 drugs (15 x 3 = 45 replicates).
- Total of 45 replicates for the study
8 Study Controls
8.1 Positive Control Procedure
Perform the positive control test by inoculating the syringe barrel with the drag, and wipe sample it.
8.2 Negative Control Procedure
This step is not applicable because ChemoGLO is a validated process that does not require a negative sample to be generated for validation purposes.
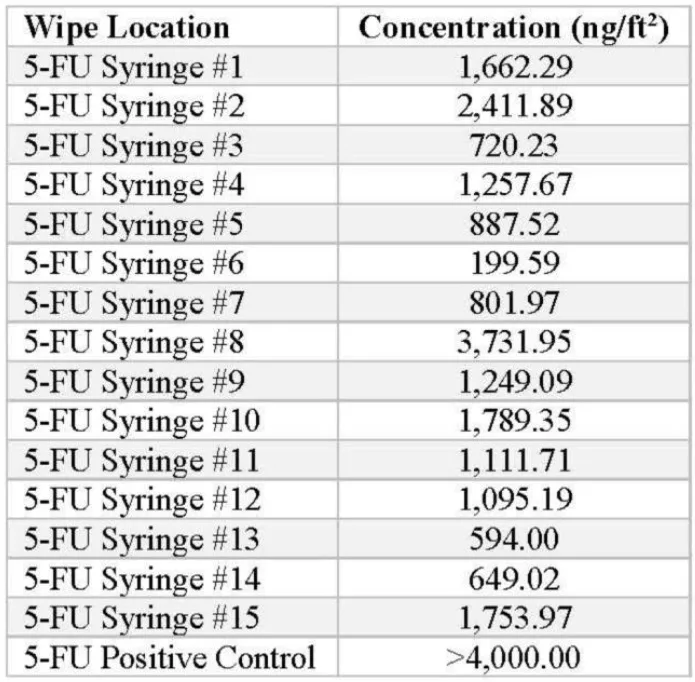
The average of the positive detections from the 15 5-FU syringes is 1,327.70 ng/syringe, demonstrating significant concentrations of the drug being detected.
9 Results
5-Fluorouacil 2.5 grams Fresenius Kabi (50 mL vial)
5-FU IV bags were prepared, and the syringe barrels were tested for the presence of contamination. The following concentrations were detected by the ChemoGLO wipe kit:
Cyclophosphamide 1 gram Sandoz (50 mL vial)
Cyclophosphamide IV bags were prepared, and the syringe barrels were tested for the presence of contamination. The following concentrations were detected by the ChemoGLO wipe kit:
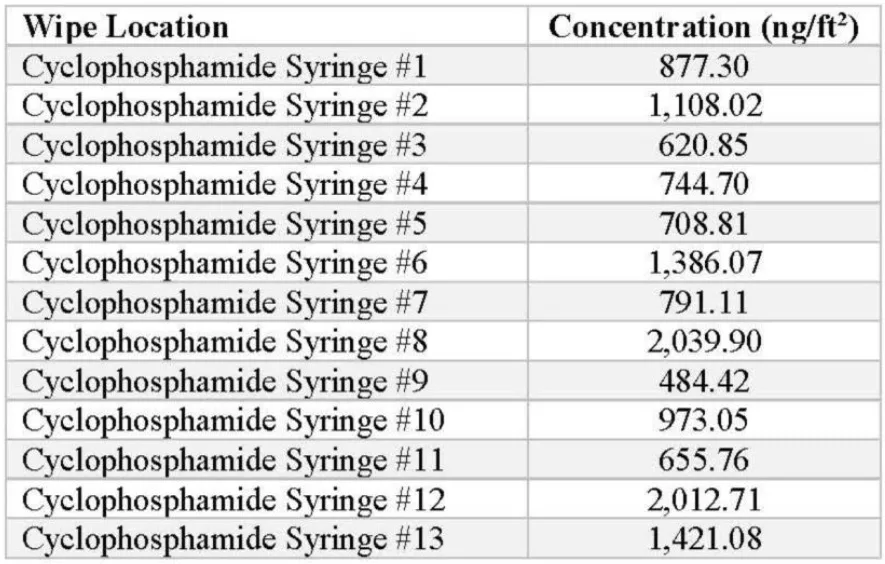

The average of the positive detections from the 15 cyclophosphamide IV syringes is 1.074.75 ng/syringe, demonstrating significant concentrations of the drug being detected.
Ifosfamide 3 grams Baxter (60 mL vial)
Ifosfamide IV bags were prepared, and the syringe barrels were tested for the presence of contamination. The following concentrations were detected by the ChemoGLO wipe kit:
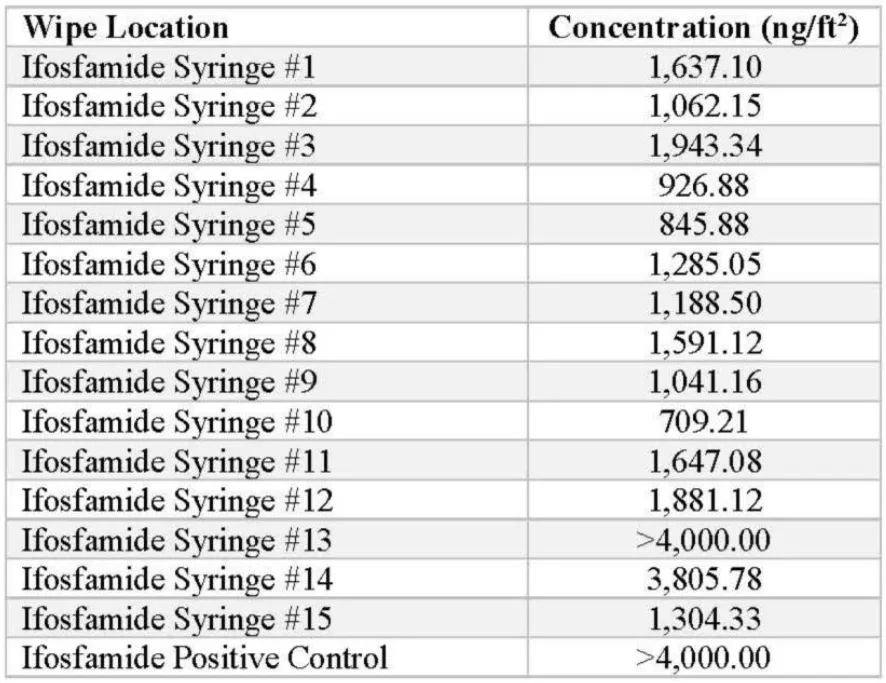
The average of the positive detections from the 15 ifosfamide syringes is 1,700.04 ng/syringe, demonstrating significant concentrations of the drug being detected.
10 Conclusions
When comparing the averages of detected concentrations of the 3 drugs (1,327.70 ng/syringe, 1,074.75 ng/syringe, and 1,700.04 ng/syringe) and in view of highest result exceeding the 4,000 ng/syringe measurement limit, these are all considered high and are of concern if they would be released into the environment or be exposed to a healthcare worker. The results of this study establish evidence for HD contamination of the inner walls of regular syringes exposed to the environment. The testing included only a single transfer of drug from vial to IV bag. Additional investigation is suggested with multiple transfers and extended duration of use that may further increase the potential for exposure. There appears to be a variation of concentrations between the different drugs (ifosfamide average is 58% higher than 5FU), indicating varying behavior of individual HDs due to tire unique physical and chemical properties of each drug. Identifying ways to reduce or contain these concentrations to eliminate this route of exposure is important for healthcare and environmental safety.