Keywords
Hazardous drugs, medication compounding, National Institute for Occupational Safety and Health (US), occupational exposure, protective devices.
Date received: 9 January 2018; accepted: 28 May 2018
Introduction
Exposure to hazardous drugs in the workplace can lead to serious health risks, and these risks increase with frequency of exposure; therefore, it is crucial to limit this with the proper protective equipment. The risks associated with the compounding and administration of hazardous drugs are well known and documented.1–9
In 1981, the Occupational Safety and Health Administration cited a northern California hospital for failure to provide protection to pharmacists preparing chemotherapy.10 This later led to the creation of a program containing recommendations for handling cytotoxic drugs in hospital11 In September 2004, The National Institute for Occupational Safety and Health (NIOSH) revised the previous 1990 American Society of Health-System Pharmacists (ASHP) definition of hazardous drugs to include drugs that exhibit one or more of the following six characteristics in humans or animals: carcinogenicity, teratogenicity or other developmental toxicity, reproductive toxicity, organ toxicity at low doses, genotoxicity, and structure and toxicity profiles of new drugs that mimic existing drugs determined hazardous by the above criteria.12,13
The main routes of exposure are inhalation of aerosolized drug, ingestion, injection, and dermal absorption.13 To minimize this exposure and protect the worker, hazardous compounding takes place in a biological safety cabinet with vertical airflow hood and external exhaust. Data indicate that healthcare workers who used safe handling precautions such as gloves, gowns, and goggles were less likely to be exposed to hazardous drugs during compounding.14 However, a 1999 study that examined surface contamination with antineoplastic agents in six cancer treatment centers in Canada and the United States found measurable amounts of antineoplastic agents in 75% of pharmacy samples and 65% of the administration samples.15 Sample sites included biological safety cabinets, countertops, and floors in and adjacent to preparation areas. Widespread surface contamination increases the risk of skin contact and dermal absorption of hazardous drugs.13
Since the publication of the 2004 NIOSH Alert, the use of closed system transfer devices (CSTDs) for hazardous drug preparation has increased in United States hospitals. The 2011 ASHP national survey of pharmacy practice found that 41% of hospitals used CSTDs for safe handling of hazardous drugs.16 Closed systems limit the potential for generating aerosols and exposing workers to hazardous drugs, and the literature documents a decrease in drug contamination of surfaces when a CSTD is used.17–20 Previously, the General Chapter: USP <797> Pharmaceutical Compounding – Sterile Preparations, contained minimal information for safety and handling of hazardous drugs. In February 2016, USP released a new General Chapter: USP <800> Hazardous Drugs – Handling in Healthcare Settings. The recently published General Chapter <800> guideline recommends the use of CSTDs for hazardous compounding and requires them for administration.21 Several CSTD brands exist in the marketplace, all classified as Class II medical devices, leaving multiple options for pharmacy and nursing to select. In 2012, the US Food and Drug Administration (FDA) began issuing 510(k) clearances under the product code “ONB” that was specific to CSTDs. However, there are no set performance standards for companies to follow to obtain these 510(k) clearances.22 To determine whether various CSTD products available in the market are truly closed systems, several studies have been performed that look at efficacy of connectors with drug surrogate to aid in product selection.23–27 Thus, it is important to identify a test and process that is consistent and allows comparisons across all current and future CSTDs in their ability to be leak-proof and airtight.
NIOSH released a proposed protocol in 2015 to evaluate the vapor or liquid containment abilities of CSTDs.28 Due to the increasing number of CSTD products since the initial NIOSH alert in 2004, the development of a performance test protocol was necessary to create standards for drug containment. In addition, developing a universal protocol will help expand validation of a CSTD beyond the current FDA 510(k) product clearance system to help healthcare systems make informed decisions. The protocol focused on simulating specific compounding and administration tasks performed by healthcare workers. Isopropyl alcohol 70% was the challenge agent used due to its safety, ease of manipulation, and detectability.28 The high vapor pressure of isopropyl alcohol challenges CSTDs that claim to have a truly closed system. Unlike isopropyl alcohol, hazardous drugs are likely to settle out of the air onto surfaces if the CSTD does not contain them.
This current study followed the methodology outlined by the 2015 proposed NIOSH protocol that challenges the vapor containment abilities of CSTDs. Data were generated for the following CSTD brands: ICU Medical’s ChemoClave, ICU Medical’s ChemoLockTM, Equashield’s Equashield, B. Braun and Teva Medical’s OnGuardTM with Tevadaptor, BD Carefusion’s PhaSealTM, and BD CareFusion’s SmartSiteTM VialShield.
Table 1
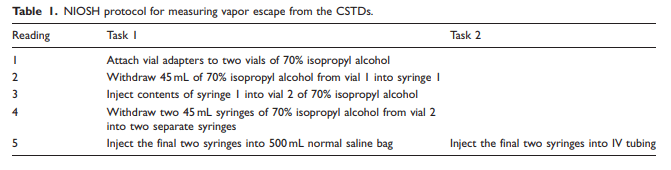
Table 2
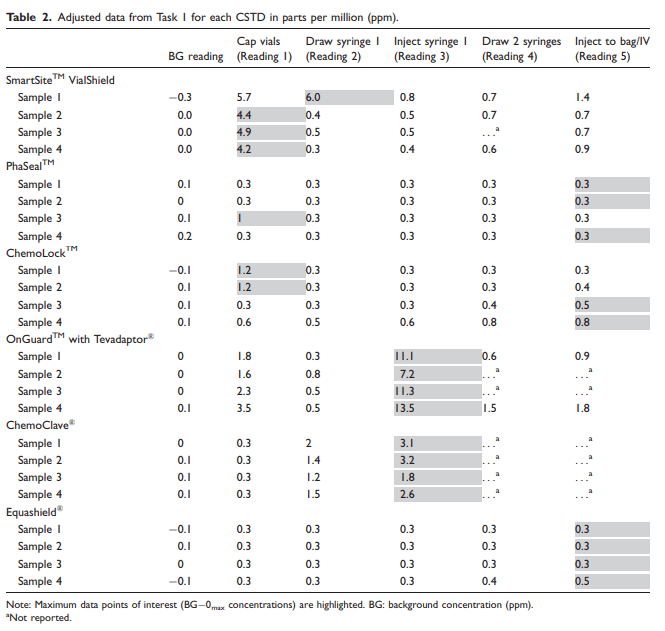
Table 3

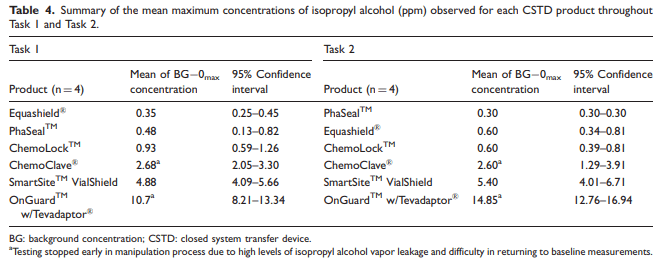
Table 4
Methods
Study objectives and procedures
The primary objective of this study was to challenge the ability of six different CSTD brands to prevent leakage of vapor from vials during intravenous (IV) compounding and administration, determining their ability to function as closed systems. The NIOSH draft protocol (CDC-2015-0075-003) was utilized to evaluate each CSTD system during compounding (Task 1) and administration (Task 2) with 70% isopropyl alcohol as the challenge agent.28
All procedures were performed in accordance with the 2015 proposed NIOSH protocol. The environmental test chamber used was constructed from a Secador Techni-dome 360 vacuum desiccator as described in the NIOSH protocol. Vapor of isopropyl alcohol that escaped during the test manipulations was measured by a Thermo ScientificTM MIRAN SapphIRe XL Infrared Analyzer model 205B-XL.28
The study involved simulation of dose preparation, as described in the protocol, with four samples for each CSTD product. In Task 1, the technician added 90 mL of isopropyl alcohol, using two 45 mL transfers from two 60 mL syringes and two 50 mL vials, to a 500 mL normal saline IV bag. The CSTD components evaluated under this task included one bag adapter, two vial adapters, and two syringe adapters. In Task 2, the technician prepared a 45 mL dose of isopropyl alcohol in each of two 60 mL syringes and injected each syringe into the Y-site of the IV tubing, simulating an IV push. The CSTD components evaluated under this task included two vial adapters, two syringe adapters, one bag adapter, and one IV port adapter.
Vapor levels were recorded for Task 1 and Task 2 after each of the following steps: attach vial adapters to two vials of 70% isopropyl alcohol (Reading 1), withdraw 45 mL of 70% isopropyl alcohol from vial 1 into syringe 1 (Reading 2), inject contents of syringe 1 into vial 2 of 70% isopropyl alcohol (Reading 3), withdraw two 45 mL syringes of 70% isopropyl alcohol from vial 2 into two separate syringes (Reading 4), and inject final two syringes into 500 mL normal saline bag for Task 1 or IV tubing for Task 2 (Reading 5). The highest detected amount of isopropyl alcohol released was recorded after five pre-specified steps during manipulations for each device, giving a total of 24 unique data points for each task, four per CSTD brand. This is summarized in Table 1.
Study evaluation and measurements
Vapor release was measured with the Thermo ScientificTM MIRAN SapphIRe XL Infrared Analyzer, and measurements were gathered in real time after each step of the process. The highest data point recorded for each sample was used in the analysis. Readings below 0.3 parts per million (ppm) were considered below the detection limit of the equipment. Data points were adjusted for background (BG) vapor concentration and adjusted for the limit of detection of the equipment per the NIOSH protocol. BG concentrations were recorded prior to each test run over a period of 5 s. If the average BG concentration was below the limit of detection of the equipment, then no BG correction was performed. If the average BG concentrations were over the limit of detection, this value was subtracted from each data point observed. If any BG-adjusted data points were below 0 ppm, their value was adjusted to 0 ppm. The maximum data point out of the five readings recorded was the metric of interest for each test run. If this value was under the limit of detection, then it was substituted by 0.3 ppm. For Task 1 and Task 2, there were four metrics of interest corresponding with the maximum detection for each test sample. The performance threshold for successful containment of isopropyl alcohol vapor was 1.0 ppm based on the calculated limit of quantification as described in the NIOSH protocol.28 Therefore, a CSTD failed to effectively contain vapor if the 95% confidence interval contained greater than or equal to 1.0 ppm. In this study, testing for individual samples was ended prematurely if a concentration significantly over 1.0 ppm was detected as there was certainty that the device had significant leakage during the set of manipulations.
Results
Data were collected over a two-day period. A total of eight samples were tested for each of the six CSTD brands, with four samples tested per task. Each sample had a total of five readings recorded, which corresponded with specific steps in the manipulation process. The data point of interest for each sample was the maximum reading of isopropyl alcohol from the detector after adjustments for BG concentrations and zero corrections, shown in Tables 2 and 3. The mean and 95% confidence interval of the mean were calculated for the maximum adjusted concentrations of isopropyl alcohol (ppm) observed for each CSTD product during Task 1 and Task 2, shown in Table 4.
Average values less than 1.0 ppm indicated that the CSTD successfully contained isopropyl alcohol vapor per the NIOSH protocol. For Task 1, two CSTDs successfully contained isopropyl alcohol vapor per NIOSH protocol, and for Task 2, three CSTDs successfully contained the isopropyl alcohol vapor.
Discussion
In this study, only two CSTDs performed as truly closed systems during both compounding and administration manipulations, measured by the release of isopropyl alcohol vapor using the Thermo ScientificTM MIRAN SapphIRe XL Infrared Analyzer. The 2015 NIOSH draft protocol proposed the use of the Thermo ScientificTM MIRAN SapphIRe XL Infrared Analyzer to quantitatively measure isopropyl alcohol that escaped during the test manipulations because the instrument is capable of providing a specific response to isopropyl alcohol, has a moderately low detection limit of 0.3 ppm, and is commonly available in the industry. A BG0max concentration of 0 ppm would indicate a truly closed system; however, a substitute zero of 0.3 ppm was utilized based on the limit of detection of the equipment.
From the limit of detection, the limit of quantification at which analytes can be definitively quantified was calculated to be 1.0 ppm. NIOSH claims that the false negative rate above the limit of quantification is negligible, ensuring that leakage measured during this study does indeed represent true leakage from the CSTD’s manipulations.28 If a CSTD product reached a concentration significantly over 1.0 ppm, the testing for that particular manipulation was ended prematurely because there was significant leakage. For this reason, data presented in this study should be used to determine if a CSTD product is truly a closed system, but cannot be used to rank the CSTD products in their ability to maintain a closed system.
This protocol tested two types of CSTDs: physical arrier and air filtration devices. However, the 2015 IOSH protocol draft only claims to be applicable or CSTDs of the physical barrier type and did not ake into consideration the air filtration devices.28 Air-cleaning or filtration technology CSTD systems re only worker protective if they are used to compound drugs with no vapor generating potential. sopropyl alcohol has a higher vapor pressure than hazardous drugs currently used therapeutically, which ould result in a falsely high detection of vapor that ould not be representative of vapor release when compounding with actual drug. This needs to be considered hen evaluating the results of air-cleaning or filtration technology CSTDs. The reason these were introduced into the test is that the initial idea by NIOSH was that this performance test would cover all device types and that these devices are marketed and sold against the physical barrier CSTDs. The 2016 NIOSH draft protocol addresses the limitations of the 2015 protocol by developing an additional test protocol for air-cleaning systems that includes a definitive surrogate agent that more closely resembles the actual vapor pressures of a hazardous drug. Potential surrogate compounds that are considered better based on criteria including high vapor pressure, solubility of at least 0.10%, liquid at room temperature, and low toxicity. The surrogate compounds under review for incorporation into the protocol include dimethyl sulfoxide, trimethyl phosphate, tetramethylurea, triacetin, propylene glycol, tetraethylurea, triethyl phosphate, 2-phenoxyethanol, and tripropyl phosphate.29 However, neither protocol has been finalized as NIOSH continues to investigate. Sites that utilize air-cleaning technology CSTDs could routinely conduct wipes to detect contamination on the surfaces where hazardous drugs are compounded and administered, a recommendation within USP <800>. This could help evaluate the effectiveness of containing hazardous drugs.
Conclusion
To improve employee safety in chemotherapy preparation, CSTDs that demonstrate no leakage should be the preferred choices. In this study, both PhaSealTM and Equashield products proved to be adequately closed in both Task 1 and Task 2, while ChemoLockTM showed to be closed only in Task 2. All other products failed both tasks when measuring for isopropyl alcohol vapor release.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Stephen F Eckel is a founder of ChemoGLO, LLC