The ongoing battle against cancer has seen a paradigm shift with the advent of CAR-T cell therapy, a revolutionary approach that utilizes the patient’s own immune system to fight cancer. This innovative treatment, coupled with gene therapy, is transforming the landscape of cancer care.
Unveiling CAR-T Cell Therapy
Chimeric Antigen Receptor T-cell (CAR-T) technique was designed to augment the body’s natural defenses by equipping T cells, the soldiers of the immune system, with engineered receptors known as CARs. These receptors enable the T cells to recognize and attack specific cancer cells, thereby providing a targeted approach to cancer treatment.
CAR-T cell therapy has proven to be a game-changer in treating certain types of blood cancers, such as B-cell acute lymphoblastic leukemia and diffuse large B-cell lymphoma, that had previously shown resistance to conventional treatments1.
Manufacturing Process of CAR-T Cell Therapy

The production of CAR-T cells involves several intricate steps, each requiring meticulous precision to ensure the utmost safety and efficacy of the final product. The process commences with the collection of T cells from the patient’s blood through a procedure called leukapheresis, where a specialized machine separates the desired cells.
These collected cells, which play a crucial role in the immune system, are then transported to a laboratory, where they undergo a series of genetic modifications to express Chimeric Antigen Receptors (CARs) on their surface. This genetic engineering process involves precisely inserting the CAR gene into the T cells, allowing them to recognize and target specific cancer cells. Subsequently, the modified cells are cultured and expanded in the lab, undergoing rigorous quality control checks to ensure their purity, potency, and safety.
Once these quality standards are met, the final product, consisting of the genetically modified CAR-T cells, is prepared for infusion back into the patient, where they can potentially combat the cancer cells with enhanced specificity and effectiveness.
Contamination Risks in Cell Therapy Manufacturing: Safeguarding Patients and Preserving Quality
Contamination can occur at various stages of cell therapy manufacturing, such as during genetic modification, cell expansion, or product formulation. Even a small presence of external contaminants, such as microorganisms or particles, can undermine the therapeutic value of the treatment and pose significant risks to patients.
Complications arising from contamination may evade standard quality control measures, leading to the release of a contaminated batch. If administered to patients, this could result in adverse effects, reduced treatment effectiveness, or even serious harm. Moreover, such incidents could have far-reaching consequences for the manufacturer, including financial burdens, legal challenges, damage to reputation, and ethical concerns.
Role of Closed System Devices
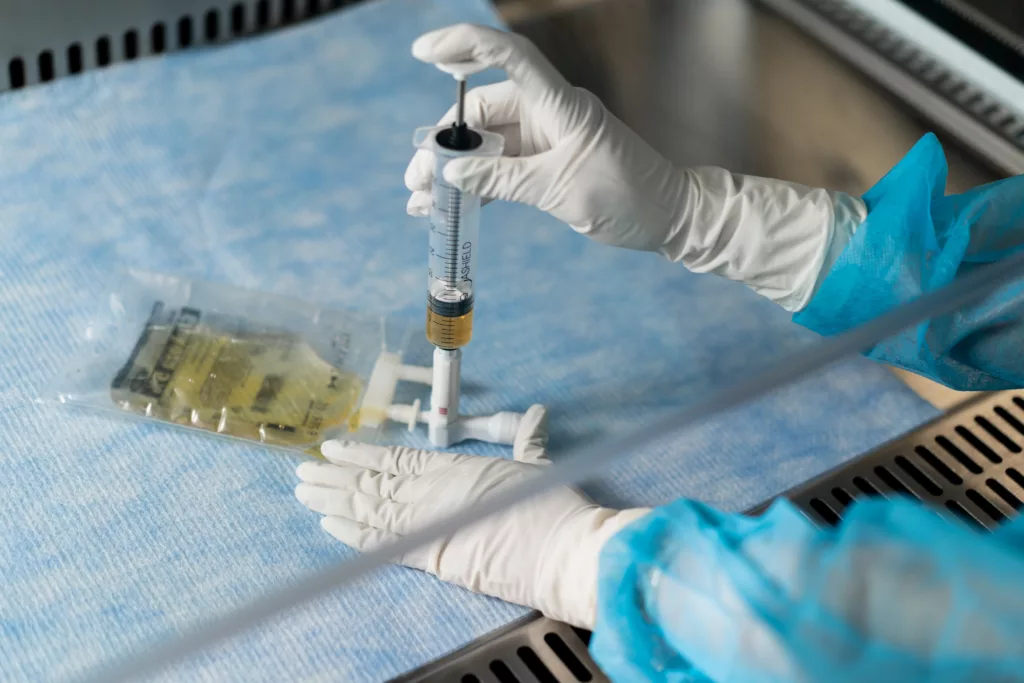
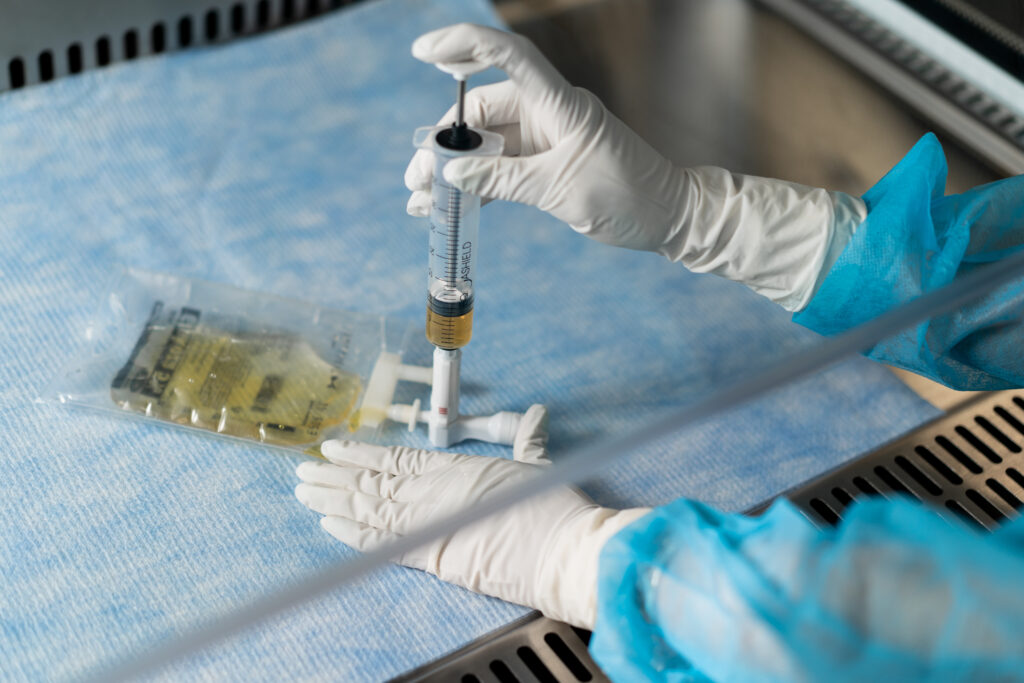
CSTDs play an important role in the manufacturing and quality control processes of CAR-T cell therapy, improving sterility, decreasing batch failure rates and improving overall process efficiency. These devices prevent the introduction of contaminants into the system and restrict the escape of hazardous drugs or vapors, ensuring a safe and controlled working environment2.
Throughout the therapy manufacturing process, from transportation to quality control, air removal, and sampling, Closed System Transfer Devices (CSTDs) play a role in reducing contamination risks. Let’s take a closer look at how these devices effectively safeguard the integrity of the therapy. CSTDs offer a controlled environment for testing and analysis. By maintaining a closed system, these devices minimize the chances of external contaminants infiltrating the samples, thereby ensuring accurate and reliable results. This is particularly crucial as even the slightest contamination can skew test outcomes and lead to erroneous conclusions.
By incorporating Closed System Transfer Devices (CSTDs), CAR-T companies can significantly reduce the potential for contamination throughout the manufacturing process of CAR-T cell therapy. These devices serve as a reliable defense mechanism, contributing to the safety, effectiveness, and integrity of the end product.