BACKGROUND
- The risks associated with compounding and administration of hazardous drugs
(HD) has been evaluated and documented in the literature. - USP 800 guidelines released earlier this year:
- Require use of a closed system transfer device (CSTD) for HD administration.
- Recommend the use of a CSTD for HD preparation.
- Since the development of CSTDs, several options exist for HD preparation and administration. There is limited data comparing all of the products in one study.
PURPOSE
- The purpose of this study is to determine how 6 different CSTDs that are marketed as leak-free behave when tested with actual drug.
METHODS
- To assess the integrity of CSTD connectors, 6 types of CSTDs were tested for leakage for up to 3 connections.
- Using sixty 5-fluorouracil (5-FU) vials, each fitted with one CSTD vial access device, a total of 10 samples were obtained for each of the 6 CSTD types.
- A 10 mL syringe was connected to the vial and 7 mL was withdrawn using a Pull-Push-Pull method to simulate air bubble removal. The vial was inverted upright to re-inject 5 mL into the vial and then the connectors were disconnected. The syringe was then reconnected to the vial and the remaining 2 mL of drug was injected in
to the vial. This was repeated two more times with the same CSTD. - Testing Group 1 (TG1) investigated leakage from the vial (V) and syringe (S) during the 2nd and 3rd connection.
- Testing Group 2 (TG2) investigated leakage during the 1st and 3rd connection.
- Leakage of the device was evaluated qualitatively using litmus paper to assess if there was visible leakage on
the vial and syringe connector
RESULTS
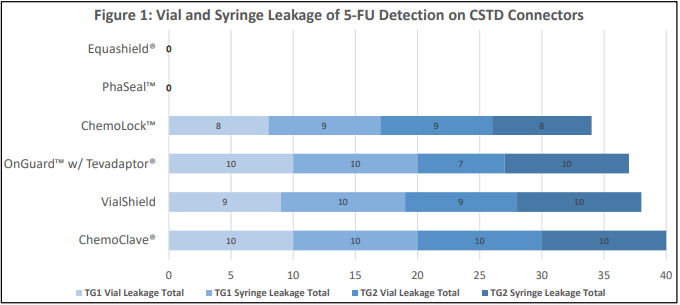
- A total of 120 samples were prepared and assessed for litmus paper discoloration from either the syringe or vial connector of 6 CSTDs.
- The negative control was performed by wiping litmus paper on the 5-FU vial stopper and resulted in no color change. The positive control was performed by placing one droplet of drug onto the litmus paper and resulted in color change
CONCLUSIONS
- Out of 6 closed system transfer devices, 4 had detectable leakage while 2 had no visible leakage.
- Equashield® and PhaSeal™ are the two CSTDs that demonstrate a completely closed system.
- To improve patient outcomes and employee safety in chemotherapy preparation, CSTDs that demonstrate no leakage should be the m preferred choices.
- Limitations: Two technicians alternated testing for each different CSTD; however, both were trained in the same way. The litmus paper may have been wiped on the syringe and vial with varying force between two technicians.
FIGURE 1. Separate syringes and vials were fitted with the CSTD being tested before manipulation of 5-FU.

FIGURE 2. Example of the table used to record leakage of the CSTD from the vial (V) or syringe (S).
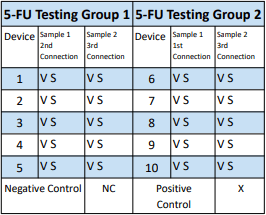
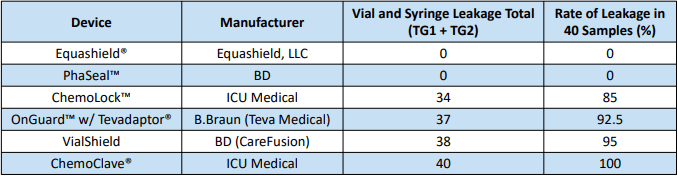
FIGURE 2. ChemoClave® demonstrating leakage in both the syringe and vial connector during a sample run.
FIGURE 3. Equashield® demonstrating no leakage in the syringe and vial connector during a sample run