Introduction
Acute and long-term health risks have been associated with occupational exposure to antineoplastic agents, including infertility, birth defects and some types of cancer.1–4 Human and veterinary health care workers risk exposure to hazardous drugs during preparation and administration through leakage or accidental spills. In the USA, an estimated 500,000 veterinary health care workers including veterinarians, technicians, maintenance personnel and office staff are potentially exposed to hazardous drugs at their workplace. 5 Safe handling practices implemented in the 1980s, including the use of personal protective equipment (PPE) and ventilated biologic safety cabinets (BSC), have helped to reduce but not eliminate the occupational exposure of health care workers who handle these agents. 6,7 Even with use of BSC, traces of cytotoxic drug contamination have been detected in pharmacies where the drugs are prepared8–10, and measurable concentrations of hazardous drugs were documented in the urine of health care workers who prepared or administered them, even after safety precautions had been employed.1,4,9
In 2004, the National Institute for Occupational Safety and Health (NIOSH) issued an alert recommending the use of closed system transfer devices (CSTD) in combination with PPE and BSC. 1 NIOSH defines a CSTD as a drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and escape of hazardous drug or vapour outside the system, thereby preserving the sterility of the product while preventing the escape of hazardous drugs. 1 Studies have demonstrated that while traditional methods of drug preparation are associated with high levels of leakage, nearly complete containment of liquids and aerosols is possible using CSTD. 6,8–9,11–12,14–16
Two CSTD marketed to veterinary oncologists at the time this study was designed, Equashield™ (Equashield LLC, Port Washington, NY, USA) and PhaSeal® (BD Medical, Franklin Lakes, NJ, USA), were chosen for comparison in this study. Both systems use double membrane connectors for drug transfer to ensure dry connections and were chosen because of demonstrated efficacy in preventing leakage. PhaSeal® was the first CSTD to be commercially available and, as such, has been the system used most frequently in studies of CSTD. PhaSeal® reduces hospital environmental contamination by 65–100% compared to conventional antineoplastic drug preparation methods.6,11–12,14–16 Similarly, no environmental contamination was detected in samples obtained one year after implementation of Equashield™ at an ambulatory cancer chemotherapy infusion center.6
The PhaSeal® system (Fig. 1) includes three main components: the injector, the protector and the connector. In addition, there are a variety of specialized administration products and an assembly fixture for attaching protectors to drug vials. Injectors attach to Luer lock syringes of any volume. Protectors attach to drug vials and use a sealed expansion chamber to maintain neutral pressure during drug reconstitution. Connectors are connection devices used in all patient or infusion connections. The Equashield™ system (Fig. 2) also has three main components: the syringe unit, vial adaptor and a variety of adaptors for fluid injection or withdrawal. The unique syringe is airtight and contains two chambers, a distal chamber for air and a proximal chamber for liquid. Air contained behind the plunger of the syringe is transferred into the drug vial when liquid drug is withdrawn into the syringe. One, 3, 5, 10, 20, 30 and 60 mL syringes PhaSeal® and no CSTD and (2) to compare the costs of Equashield™ and PhaSeal®.
Materials and methods
Study subjects The study protocol was approved by the University of Georgia Institutional Review Board (IRB) for use of human subjects. Veterinary technicians (VT) from 16 private practice and university hospitals were identified through telephone calls or e-mail communication with veterinary oncologists and recruited via e-mail using an IRB-approved recruitment letter. Oncologists were selected for initial contact based on CSTD use for chemotherapy administration in their practice (yes/no and if yes, Equashield™ or PhaSeal®). The intent was to recruit a total of 45 VT: 15 with experience using Equashield™, 15 with experience using PhaSeal® and 15 with no experience with CSTD. Inclusion criteria were: (1) Participating VTs’ primary job responsibilities included cancer chemotherapy administration and (2) VT were required to sign an IRB-approved informed consent form prior to participation. The only incentive for participation was the opportunity to gain experience with the two CSTD systems.
Data collected about VT included licensing/registration status, specialization as a Veterinary Technician Specialist in Oncology (yes/no), years of experience as a VT, years of experience administering chemotherapy to veterinary patients, and experience with CSTD (none or had used Equashield™, PhaSeal® both, or another system). This information was recorded on a standardized data collection sheet developed for this study (Appendix S1).
Timed simulated administration of chemotherapy
Timed simulated administration of chemotherapy was performed at the institutions where the VT were employed. Prior to performing the simulations, all VT (including those with prior experience with CSTD) watched the instructional recordings provided by the manufacturers for each CSTD [http://www.bd.com/pharmacy/PhaSeal/flash/guide/index.html (eLearning Module titled, Administration – from syringe to patient) and http://www.equashield.com/using_equashield.php (Video Demonstration 5, titled, IV Push or Bolus Through a Luer Lock Port)] as they would in preparation for using these systems in clinical practice. Both CSTD were provided for manipulation while watching the instructional recordings. After watching the training recordings, each VT performed simulated IV administration of chemotherapy and saline flush to a dog 30 times: 10 times each using Equashield™, PhaSeal® and no CSTD. To account for a possible learning effect over the course of the simulations, the order of use of each CSTD or no CSTD for simulated chemotherapy administrations by each VT was assigned according to a Latin square randomization method. Technicians were grouped into three experience groups based on whether they had used Equashield™ or PhaSeal®, or had administered chemotherapy with no CSTD, and the randomization of the order in which Equashield™, PhaSeal® or no CSTD was used by each VT was within each experience group. The order was specified in the data collection sheet provided for each VT.
Simulated ‘chemotherapy’ for IV administration consisted of 4 mL of water administered by IV push into a model developed for this study (Fig. 3). Administration was preceded by flushing with 3 mL and followed by flushing with 5 mL of simulated ‘saline’ (water). The model consisted of a 22-g IV catheter (SURFLO® ETFE I.V. Catheter, Terumo Medical Corporation, Somerset, NJ, USA) placed in tubular foam pipe insulation (Frost King®, Thermwell Products, Mahwah, NJ, USA) approximating the size and shape of a canine antebrachium. The catheter was capped with a T-connector (Abbott Animal Health, Abbott Park, IL, USA) and secured with white medical tape. Technicians wore chemotherapy gloves (CHEMO PLUS™, Covidien, Mansfield, MA, USA) during the simulation. All materials needed for the study were supplied in kits assembled by the investigators and mailed to each participating site.
Observers positioned and stabilized the model and timed administration using a stopwatch. There was no requirement for a single observer for all VT at each facility, however, to avoid confounding due to variability in timing and model positioning, for each VT, a single observer timed all episodes of simulated chemotherapy administration. Timing started with the VT’s hands up before flushing the IV catheter. Steps included in the timing were flushing the IV catheter, administering simulated chemotherapy, and flushing the catheter again. Timing stopped when the VT raised his/her hands up after disconnecting the final flush and setting the syringe to the side. Administration time was recorded on a standardized data collection sheet (Appendix S1).
Evaluation of systems by VT
After performing 10 simulated chemotherapy administrations with each system (i.e. no CSTD, with Equashield™ and with PhaSeal®) in the order of his/her randomized assignment, each VT rated the EOU and likelihood that he/she would recommend each system to a colleague. Ten centimetre visual analogue scales (VAS) were used for VT ratings (Appendix S1). Location of the mark was translated into a numerical value from 0–10 with 0 indicating impossible to use and 10 indicating easiest possible to use, or 0 indicating would never recommend to a colleague and 10 indicating would always recommend to a colleague (Fig. 4). There was no requirement for a VT to complete all 30 simulated administrations on the same day but individual VT were required to complete all 10 simulated administrations for each individual system in one session.
Specific instructions provided to VT and observers can be found in Appendix S2.
Cost comparison
Pricing information for the two CSTD was obtained from veterinary distributors, specifically Equashield™ Medical Ltd. for Equashield™ and Avella Specialty Pharmacy for PhaSeal®. The cost of the components needed to administer a single 4-mL IV bolus was calculated for each system.
Results
Oncology technicians from 16 hospitals were recruited for participation in this study. Forty nine VT were assessed for eligibility and 46 were included (Fig. 5). The VT included in the study were from 12 sites: the University of Georgia College of Veterinary Medicine (5), Southeast Veterinary Oncology and Medicine (6), Animal Cancer Care Clinic (6), VCA Los Angeles (5), Wheat Ridge Animal Hospital (5), Hope Veterinary Specialists (4), Veterinary Cancer Group (4), BluePearl Georgia Veterinary Specialists (3), the University of Guelph Ontario Veterinary College (3), Portland Veterinary Specialists (2), Columbia River Veterinary Specialists (2), and Veterinary Oncology Services, NY (1).
At least 15 VT with experience using Equashield™, 15 with experience using PhaSeal®, and 15 with no experience using either CSTD completed the study. Some VT had experience with more than one system such that 31 (67%) reported experience with chemotherapy administration with no CSTD, 26 (57%) with PhaSeal®, and 15 (33%) with Equashield™. In addition, three had experience with another system (Tevadaptor®, Teva Medical Ltd., Netanya, Israel). Twenty-nine (63%) VTs were licensed or registered and one was certified as a Veterinary Technician Specialist in Oncology. The median reported time working as a VT was 11 years (range, 1–30 years), with one VT not reporting, and the median time reported working as a VT specifically in the field of oncology was 4 years (range, 1 month–23 years), with four VT not reporting this information. Demographic information for VT grouped according to CSTD experience is presented in Table 1.
Median times for simulated chemotherapy administration for all attempts by all VT were 24 s using Equashield™, 29.1 s using PhaSeal®, and 26.9 seconds using no CSTD (presented with upper and lower quartiles and ranges in Fig. 6a). Administration times were not significantly different using either CSTD versus no CSTD, however administrations with Equashield™ were significantly faster than with PhaSeal® (P <0.01).
Each VT used each system 10 times. The 10th administration time was faster than the first for all systems, regardless of VT past experience, suggesting an effect of learning with practice. To account for the effect of learning, we compared the 10th administration times for each system. The medians, upper and lower quartiles, and ranges are presented in Fig. 6b. There was no significant difference between PhaSeal® and no CSTD, however, administration time was significantly faster with Equashield™ than with no CSTD or PhaSeal® (P =0.0003).
As past experience could influence VT skill using a CSTD system or no CSTD, we compared overall and 10th administration times of each system among the three technician experience groups (prior experience using Equashield™, PhaSeal® or with administration using no CSTD). Results are presented in Table 2. VT experienced with no CSTD had significantly faster 10th administration times using Equashield™ than using no CSTD or PhaSeal® (P <0.0001 for both). VT experienced with EquashieldTM and VT experienced with PhaSeal® had significantly faster 10th administration times using Equashield™ than using no CSTD (P <0.002 for both).
Technicians’ VAS ratings of EOU of each system were converted to numerical values (Fig. 4) ranging from 0 to 10, with 0=impossible to use and 10=easiest possible to use. Boxplots of results are presented in Fig. 7. Neither CSTD was rated harder to use than no CSTD, and Equashield™ was rated significantly easier to use than PhaSeal® or no CSTD (median scores of 8.3, 6.3, and 6.0, respectively; P <0.002). EOU ratings based on prior experience are presented in Table 3.
VAS were also used to determine the likelihood that a VT would recommend each CSTD or no CSTD to a colleague administering chemotherapy. Results were converted to a 0–10 numerical rating, with 0=would never recommend and 10=will always recommend (Fig. 4). Boxplots of results are presented in Fig. 8. Technicians were significantly more likely to recommend Equashield™ and PhaSeal® than no CSTD (median scores of 7.7, 6.8, and 1.8, respectively) regardless of prior experience (P <0.0001). Likelihood of recommending ratings based on prior experience are presented in Table 4.
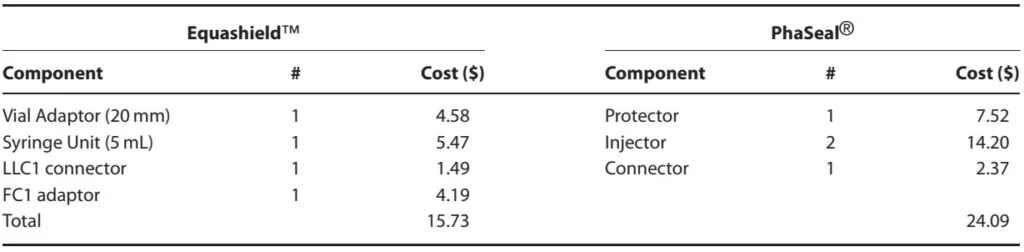
A comparison of prices for CSTD components for IV bolus of 4 mL of chemotherapy quoted on June 5, 2014 is presented in Table 5. The cost of Equashield™ components was approximately 65% that of PhaSeal® components. Equashield™ components were sold only in bulk (boxes of 30–240 units or cases of 60–480 units, depending upon the component) while PhaSeal® components were sold by the unit on a sliding price scale based on the number of units purchased (1–10, 11–49 or 50+). To make the comparison valid, the cost analysis presented in this study was based on the purchase of one box of each Equashield™ component and 50+ units of each PhaSeal® component.
Figure 1
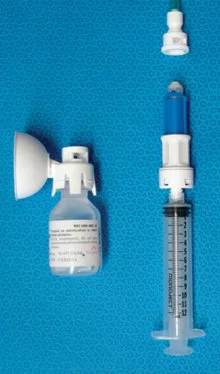
Figure 2
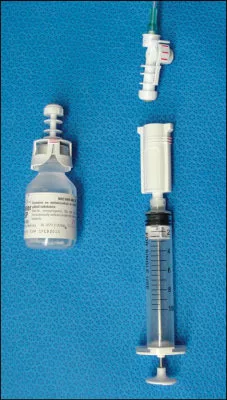
Figure 3

Figure 4
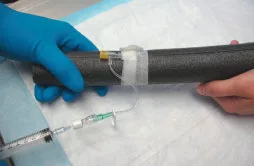
Figure 5
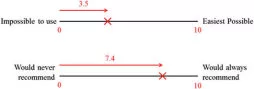
Table 1
Figure 6
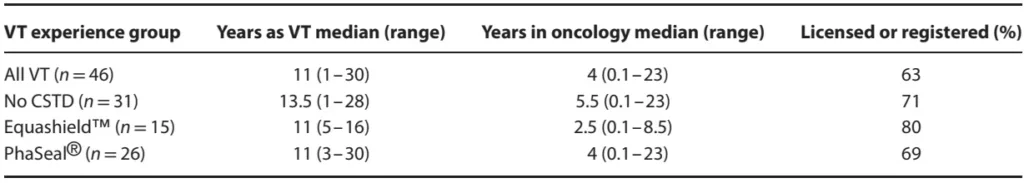
Table 2
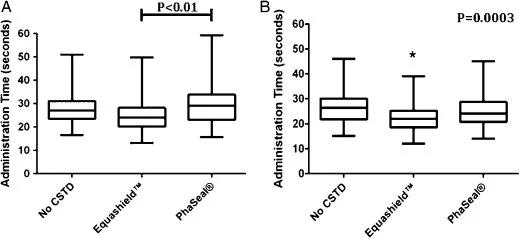
Figure 7
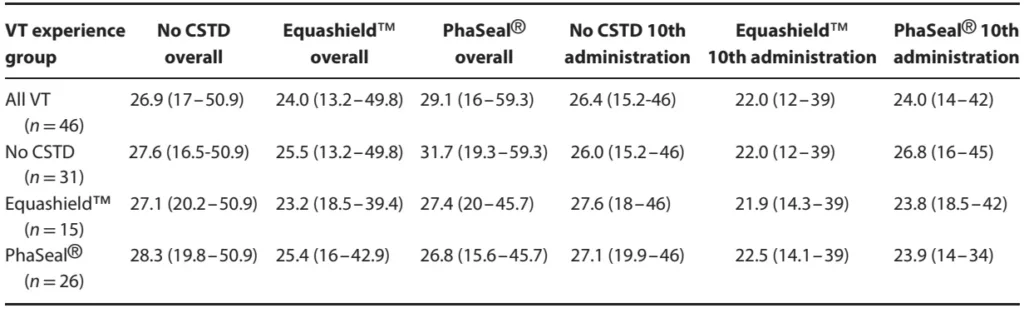
Table 3
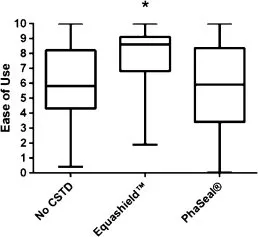
Figure 8
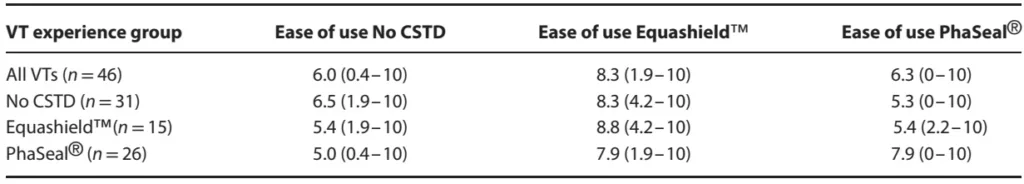
Table 4
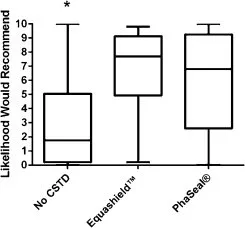
Table 5

Discussion
In 2004, the NIOSH published the alert, ‘Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings’.1 In that alert, use of closed-system transfer devices was recommended. Since then, the United States Food and Drug Administration (FDA) created a product code (ONB) for closed antineoplastic and hazardous drug reconstitution and transfer systems to ensure their efficacy and inform health care professionals. Clearance of these devices by the FDA is based on prevention of escape of hazardous drug into the environment and transfer of environmental or microbial contaminants into the drug. Our study evaluated the efficiency, EOU, and cost of two FDA-approved CSTD, Equashield™and PhaSeal®. Based on the results presented here, use of a CSTD does not increase administration time of IV bolus chemotherapy. Both systems were readily accepted and recommended over no CSTD by VT who administered chemotherapy as part of their primary job responsibilities. Equashield™ was easier to use, but neither closed system was rated more difficult to use than no CSTD. For bulk purchases, Equashield™ would be more cost effective. For an individual unit or small numbers of units, PhaSeal® would be more cost effective.
Participating VT rapidly gained familiarity and skill with CSTD use, whether or not they had previous experience with Equashield™ or PhaSeal®. Administration time became significantly faster for both closed systems as VT progressed from the first to tenth simulation. In fact, by the 10th administration, administration times were faster for Equashield™ than for no CSTD.
While administration times were fastest with Equashield™, it must be acknowledged that the 2-5s difference between median administration times would not appreciably affect case flow. For example, in a practice administering 20 chemotherapy treatments per day, this would equate to 1–2 min saved. Importantly, however, neither CSTD was less efficient than no CSTD. Our results are in agreement with two other studies of antineoplastic drug preparation using PhaSeal® that demonstrated no impact on efficiency or significant time savings.11,17 Concern for decreased practice efficiency should not deter veterinarians from adopting CSTD use.
Perceived ease of use was significantly higher for Equashield™ (median VAS rating of 8.3/10, with 10 representing easiest possible) than PhaSeal® (6.3/10) or no closed system (6/10). Ratings of 6–8.3 suggest that chemotherapy administration with either or no CSTD requires training but is reasonably easy. Participating VT were much more likely to recommend use of a CSTD than no CSTD (median VAS ratings of 7.7/10 for Equashield™ and 6.8/10 for PhaSeal® versus only 1.8/10 for no CSTD, with 10 representing ‘would always recommend’), suggesting not just a willingness to accept use of CSTD in their practice, but that VT would actually promote use of the systems.
Technicians were asked to provide comments about their VAS ratings. They reported that Equashield™ was easier to learn how to use, less bulky, and easier to connect and disconnect, but they expressed concern about the security of the connection and fragility of the components. Subjectively, VT were more comfortable with the security of the PhaSeal® connection but reported that this system was more cumbersome, difficult to connect and disconnect, and time consuming to use, at least at first. Technicians commented that they were not likely to recommend use of no CSTD for chemotherapy administration due to occupational safety concerns. It is clear that VT in oncology specialty practice are aware of the dangers of chemotherapy exposure and are interested in implementing CSTD use in their practice. This study shows that CSTD are not difficult to use, and VT acceptance is high. These concerns should not impede their implementation in veterinary practice.
A weakness of this study is that 21 of 46 VT had experience using more than one CSTD or administering chemotherapy with and without a CSTD. During recruitment, we found that some VT using CSTD had experience administering chemotherapy with no CSTD prior to adoption of CSTD in their practice and some had used more than one CSTD system. In particular, because Equashield™ was newer, there were few sites using this system and most (11/15) VT using Equashield™ had experience with another CSTD or no CSTD.
Consequently, because we would not have been able to recruit a sufficient number of participants to carry out the study otherwise, we adjusted our recruitment goal to include 15 VT currently using each system or no CSTD, irrespective of previous experience.
Another point to consider is VT participating in this study worked in oncology specialty practice so their assessments of CSTD may not be representative of the opinions of all VT.
Additional costs of using CSTD in chemotherapy administration might be a concern for veterinarians and VT-treating cancer patients. Based on our cost analysis, an additional cost of $16–25 would be incurred to administer an IV bolus of chemotherapy.This does not seem prohibitive, especially when considering the value of these devices in protecting veterinarians, staff, and owners from exposure to hazardous agents. In addition, in a study performed by Edwards et al. 2013, the use of PhaSeal® resulted in a noticeable cost saving ($700,000/year in the pharmacy studied) by allowing the unused portion of single-use vial to be salvaged, thereby cutting down on drug waste.19 The cost of Equashield™ was approximately 65% of the cost of PhaSeal®, but this system is sold only in bulk quantities. Therefore, based on cost, Equashield™ is a reasonable choice for practices with a high volume of patients receiving chemotherapy and PhaSeal® for practices treating an occasional patient with chemotherapy.
This study demonstrates that the amount of time required to administer an intravenous dose of chemotherapy is not increased by the use of a CSTD. Equashield™ is easier to use, but both Equashield™ and PhaSeal® are reasonably easy to use and are recommended over no closed system by VT working in oncology specialty practice. CSTD are not cost prohibitive. These systems should be adopted by all veterinary practices administering cancer chemotherapy.
Figure 1. PhaSeal® closed system transfer device. The three main components are the protector (left), injector (bottom right) and connector (top right). The injector contains a needle enclosed within a protective sleeve sealed by a double membrane. It attaches to a syringe or IV tubing. The protector is a drug vial adaptor with a flexible expansion bulb that is permanently attached to the vial and used for closed drug reconstitution and pressure equalization. The connector attaches to an IV catheter to provide a closed connection with the injector.
Figure 2. Equashield™ closed system transfer device. The three main components are the vial adaptor (left), syringe unit (bottom right) and LL1 Luer lock adaptor (top right). The syringe unit is enclosed to prevent drug exposure through contaminated plungers or open barrels. It has a built-in pressure equalization system and uses a double membrane system to seal connections.
Figure 3. Model canine antebrachium with cephalic vein catheter for simulated administration of chemotherapy.
Figure 4. Example interpretation of visual analogue scales (VAS) used by 46 veterinary technicians to evaluate the ease of chemotherapy administration using no closed system transfer device, Equashield™, and PhaSeal® (top) and likelihood he/she would recommend each system to a colleague (bottom). Technicians were asked to place a mark on the 10 cm scale indicating their rating. The distance from 0 to the mark was measured in cm and converted to a 1–10 numerical value.
Figure 5. Flow diagram of veterinary technicians participating in this simulation study evaluating use of no closed system transfer device (CSTD), Equashield™ or PhaSeal® for administration of cancer chemotherapy.
Table 1. Demographic data for veterinary technicians (VT) participating in a study evaluating use of no closed system transfer device (CSTD), EquashieldTM and PhaSeal ®, for cancer chemotherapy administration
Figure 6. Box plots of median administration time for all administrations (A) and for the 10th administration (B, to account for an effect of learning) for 46 veterinary technicians using no closed system transfer device (CSTD), Equashield™ or PhaSeal® for administration of a simulated IV dose of chemotherapy. Hinges represent the 25th and 75th percentiles and fences show the range of data. The bar and asterisk indicate statistically significant differences. While statistically significant, these differences were ≤5.1 s and would not appreciably affect clinical case flow.
Table 2. Median time for 46 veterinary technicians (VT) to administer simulated chemotherapy (in seconds, with range) overall (for 10 trials) and for the 10th (final) trial using no closed system transfer device (CSTD), Equashield™ or PhaSeal®. Results are presented for all participating VT and then for groups of VT based on their prior experience with each system
Figure 7. Box plots of visual analog scale (VAS) ratings by 46 veterinary technicians evaluating ease of use of no closed system transfer device (CSTD), Equashield™ or PhaSeal® in administration of a simulated dose of IV chemotherapy with each system. Ratings were converted to numerical values on a 0–10 scale with 0 indicating impossible to use and 10 indicating easiest to use. Hinges represent the 25th and 75th percentiles and fences show the range of data. Asterisks indicate a statistically significant difference. Technicians rated Equashield™ significantly easier to use than PhaSeal® or no CSTD (P < 0.002).
Table 3. Median ease of use rating (with ranges) for no closed system transfer device (CSTD), Equashield™ or PhaSeal® by 46 veterinary technicians (VT) following 10 trials with each system presented for all VT and grouped according to prior experience. Visual analog scale ratings were converted to 0–10 numerical values, with 0 indicating impossible to use and 10 indicating easiest possible to use.
Figure 8. Box plots of visual analog scale (VAS) ratings by 46 veterinary technicians describing the likelihood that they would recommend use of no closed system transfer device (CSTD), Equashield™, or PhaSeal® for administration of IV chemotherapy following 10 simulated administrations with each system. Ratings were converted to numerical values on a 0–10 scale with 0 indicating would never recommend and 10 would always recommend. Hinges represent the 25th and 75th percentiles and fences show the range of data. Asterisks indicate statistically significant differences. Technicians were significantly more likely to recommend Equashield™ and PhaSeal® over no CSTD (P < 0.0001).
Table 4. Median rating of how likely a veterinary technician (VT) would be to recommend a system to a colleague (with ranges) for no closed system transfer device (CSTD), Equashield™ or PhaSeal® by 46 VT following 10 trials with each system. Results are presented for all VT and grouped according to prior experience. Visual analog scale ratings were converted to 0–10 numerical values, with 0 indicating would never recommend and 10 indicating would always recommend.
Table 5. Cost comparison for Equashield™ and PhaSeal® closed system transfer devices for an example case requiring injection of 4 mL IV chemotherapy, including components for drawing up and injecting drug and saline flush.a
a Equashield™ is sold only in standardized bulk quantities. Costs presented here are unit costs based on the purchase of boxes of components. PhaSeal® prices are unit prices based on purchase of the same number of components as in Equashield™ boxes, however, PhaSeal® components may be purchased individually or in units of as many components as desired.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Data collection sheet for evaluation of efficiency, ease of use, and likelihood veterinary technicians would recommend use of no closed system transfer device (CSTD), Equashield™, or PhaSeal® for chemotherapy administration.
Appendix S2. Instructions provided by investigators to veterinary technicians participating in an IRB approved study evaluating efficiency, ease of use, and likelihood of veterinary technicians to recommend use of no closed system transfer device (CSTD), Equashield™, or PhaSeal® for chemotherapy administration.