Background
Medical personnel have been examining the issues of exposure to hazardous medications and prevention. Malformations, spontaneous abortions, and stillbirths have been associated with exposures to cytostatic agents. Closed-system drug transfer devices are recommended by the National Institute for Occupational Safety and Health (NIOSH) for the containment of hazardous drugs. The purpose
of this study is to examine available products utilized for drug-transfer to determine which device prevents the escape of vapor meeting the NIOSH definition of closed.
Methods
Nine drug-transfer devices were tested:
- Spiros™ Male Connector and Clave® (ICU Medical Inc.)
- Vial Adapter and Clave® (ICU Medical Inc.)
- B. Braun OnGuard™ System (Teva Medical Ltd.)
- Chemo Mini-Spike Plus™ Dispensing Pin (B. Braun Medical Inc.)
- Alaris’ Smart Site® (Cardinal Health)
- CyTwo-Fer (Baxa)
- CHEMO-AIDE (Baxter)
- Chemoprotect Spike® (Codan US Corporation)
- PhaSeal® Protector 50 & Injector Luer Lock (Carmel Pharma)
Titanium tetrachloride (TiCl4) was used to simulate gas-containing active drug. Titanium tetrachloride generates very visible smoke when it comes into contact with moisture in the air. It was placed into glass vials attached to the above transfer devices to determine which system prevents the escape of vapor.
Results / Conclusions
Only the PhaSeal® System prevented the release of titanium smoke out of the closed-system drug transfer device. Only the PhaSeal® System met the NIOSH definition of a closed-system drug transfer device.
PhaSeal® by Carmel Pharma
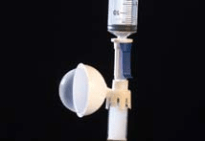
PhaSeal® by Carmel Pharma
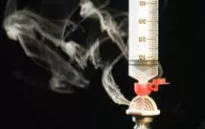
Spiros™ Male Connector and Clave® by ICU Medical Inc.

Vial Adapter and Clave® by ICU Medical Inc.
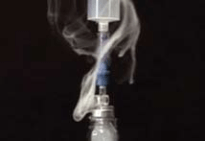
B. Braun OnGuard™ System by Teva Medical Ltd.

Alaris’ Smart Site® by Cardinal Health
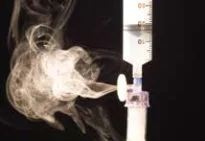
CyTwo-Fer by Baxa
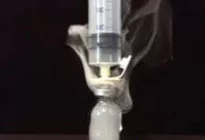
CHEMO-AIDE by Baxter
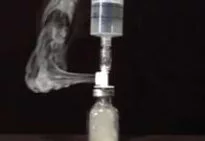
Chemoprotect Spike® by Codan US Corporation