Purpose
The United States Pharmacopeia (USP) recently published for comment chapter 800, standards on handling hazardous drugs which recommends for compounding the use of a Closed-System Transfer Device (CSTD) and proposes to mandate the use of CSTDs for the administration of hazardous drugs. This recommendation was proposed with consideration of risks for occupational exposures when handling hazardous drugs.
CSTDs are specifically designed to reduce this risk, however the use of commercially available syringes may re-introduce contamination risks during the compounding process. Four out of the five Food and Drug Administration (FDA) approved CSTD’s require the use of commercially available syringes. The fifth FDA approved CSTD manufactured by Equashield®, utilizes a proprietary closed-syringe designed to eliminate
plunger contamination.
This study was designed to evaluate the contamination potential of three syringes (BD ®, Covidien ®, Equashield ®) used for compounding hazardous drugs.
Methods
Simulated IV sterile compounding utilizing 2 gram vials of cyclophosphamide (CP) was performed with the three syringes; Covidien® and Becton Dickenson® 60mL syringes attached to the CSTD PhaSeal® and Equashield® 60mL syringes attached to Equashield® manufactured CSTD.
Prior to the study, multi-step decontamination procedures were used to ensure vials and experimental surfaces were uncontaminated per proposed USP 800 protocol.
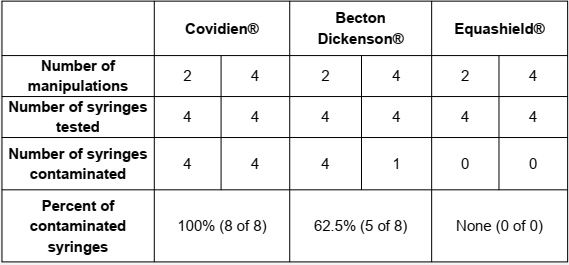
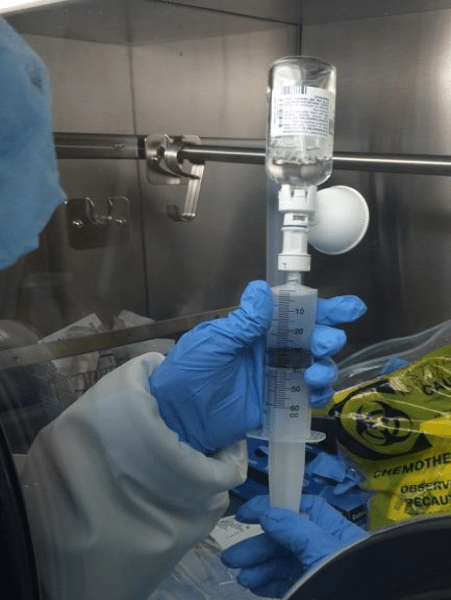
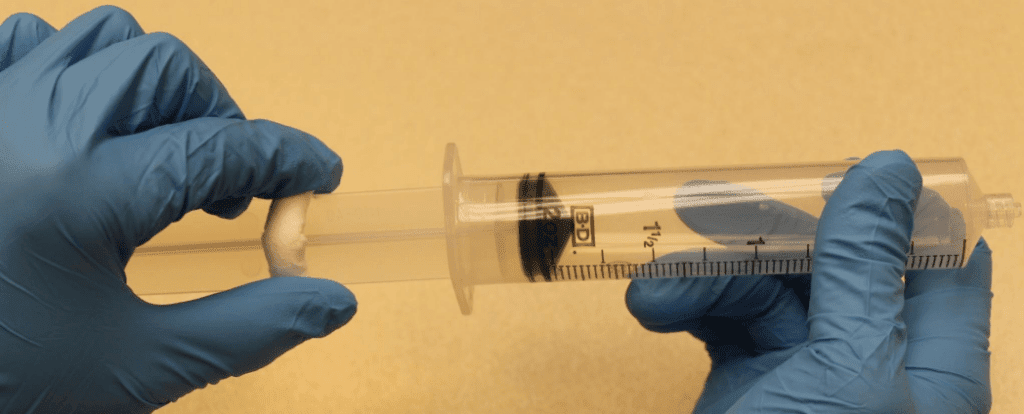
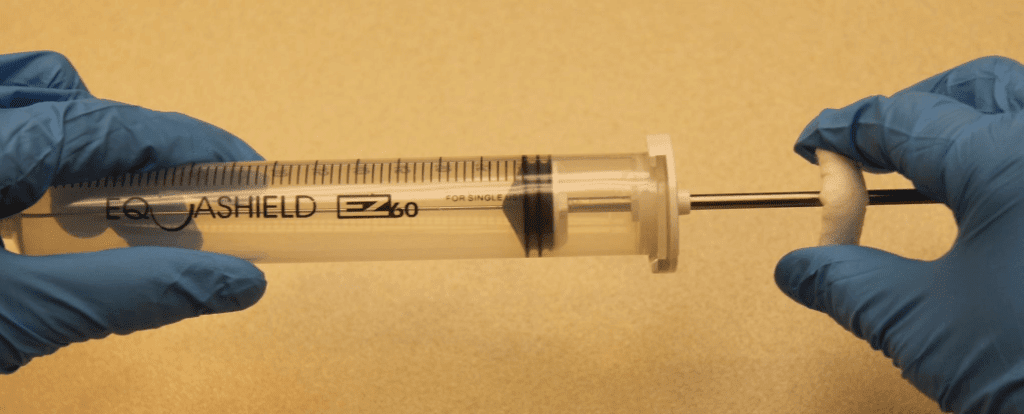
A total of 72 manipulations: 24 manipulation for each syringe in a sequence of 2 manipulations for 4 syringes and 4 manipulations for 4 syringes, Figure 1.
Extreme care was taken to minimize the potential for touch contamination of the plunger during manipulations, Figure 1.
After the last manipulation, the 60mL syringe plungers were pulled back to the 50mL position for sampling.
ChemoGlo™ (Chapel Hill, NC) sampling kits were used for quantitative evaluation of CP residue on the plunger shaft to a 10ng/ft2 sensitivity.
Wipe samples were taken from the CP vials, compounder’s gloves and from each side of the plunger shaft for each of the syringes in addition to a negative control and a positive control, Figure 2. (report available)
Results
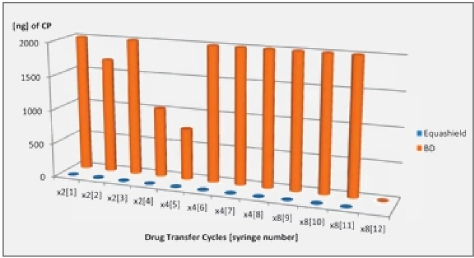
The CP vials, compounder’s gloves, and negative control samples had no CP detected while the positive CP control sample had significant CP detected (>3,500ng/ft2).
Detectable contamination occurred with all of the Covidien® syringes and 62.5% of the Becton Dickenson® syringes., Table 1
No detectable contamination was measured with Equashield® syringes.
Conclusion
The syringe represents the primary medical device used for transferring a drug from a manufactures’ container to patients via compounding. Several safety standards have been proposed to protect healthcare professionals handling hazardous drugs, such as the use of CSTDs. Currently, four of the five US approved CSTDs require the use of a commercially available syringe which may re-introduce occupational risks via plunger contamination during hazardous drug compounding. This study demonstrates the limitations of some commercially available syringes despite the use of appropriate CSTDs when performing multiple manipulations of hazardous drugs.
Caution must be considered when performing multiple manipulations with commercial syringes not designed for containment of hazardous drugs during the compounding process.
Disclosure: Authors of this presentation have the following to disclose concerning possible financial or personal relationships with commercial entities that may have a direct or indirect interest in the subject matter of this presentation.
• Fouzia Berdi and Richard Gonzalez – none to disclose
• Fred Massoomi: BD Consulting/speaking/Research Grant; Covidien Consulting; Equashield: Consulting
• Study supported by Nebraska Methodist Hospital Department of Pharmacy Services, Omaha, NE