Background
Harmful effects from workplace exposure to antineoplastic agents were first described in the 1970s.1 Noted risks of handling these agents by nurses and other healthcare personnel include damage to DNA, infertility and a possible increased risk of cancer.2–8
The use of personal protective equipment (PPE) when handling chemotherapy has been recommended by The Occupational Safety and Health Administration (OSHA) since 1986.9 Pharmacists, pharmacy technicians and nurses risk exposure to antineoplastic agents when preparing and administering these drugs. Many studies have documented surface contamination with these agents in healthcare institutions10–14 and a recent study noted that doxorubicin can penetrate nitrile gloves.15 Additionally, hazardous drugs have been found in the urine of healthcare workers who prepare or administer chemotherapy.11,13,16 PPE is therefore used during preparation and administration in order to reduce exposure during these times.
Prior studies have shown surface contamination outside of the biological safety cabinet.10–14 Healthcare workers are likely to come in contact with contaminated surfaces when not wearing PPE. Minimizing environmental contamination with antineoplastic agents is imperative to protect workers from the harmful effects of these agents.
Closed system drug-transfer devices (CSTD) can reduce exposure of health care workers to harmful agents. Numerous reports have been published that describe the effectiveness of CSTDs at decreasing surface contamination and exposure of healthcare personnel after implementation of the devices.13,14,16–21 The National Institute for Occupational Safety and Health (NIOSH)22 and The United States Pharmacopeia’s current USP 79723 standards recommend the use of CSTDs when preparing and administering chemotherapy in addition to the use of PPE.
Several CSTDs are marketed for use with cytotoxic agents. A recently published study of 22 United States hospitals noted that surface contamination was reduced significantly after the implementation of a well-known CSTD.14 However, the CSTD product used in this study has yet to be evaluated in the workplace setting. Testing of surfaces in the workplace for contamination after implementing the CSTD is important to validate the utility of the product.
Testing for surface contamination with cytostatic agents in the cancer center was completed for two reasons. An evaluation of the effectiveness of the standard method for preparing (Chemo Dispensing Pin, B. Braun Medical Inc.) and administering chemotherapy was necessary. The second reason was to evaluate whether the CSTD would decrease the level of surface contamination at various locations within the cancer center 1 year after implementation.
This study was conducted at an ambulatory cancer chemotherapy infusion center that is part of a large health-system in the Midwest of the United States. Within the center is a 21-chair infusion suite with a dedicated pharmacy preparing the chemotherapy products to be administered in the infusion suite. The cancer center has approximately 16,500 chemotherapy visits per year.
At the cancer center, the pharmacy technicians prepare all doses of chemotherapy under the supervision of the pharmacist. Pharmacy staffing consisted of two fulltime pharmacists and two full-time certified pharmacy technicians. An estimated 450 g of cyclophosphamide and 2600 g of 5-fluorouracil are prepared each year. The pharmacy has one biological safety cabinet for the preparation of all medication doses. The biological safety cabinet is a Class II Type A/B3 and has been in use for 10 years.
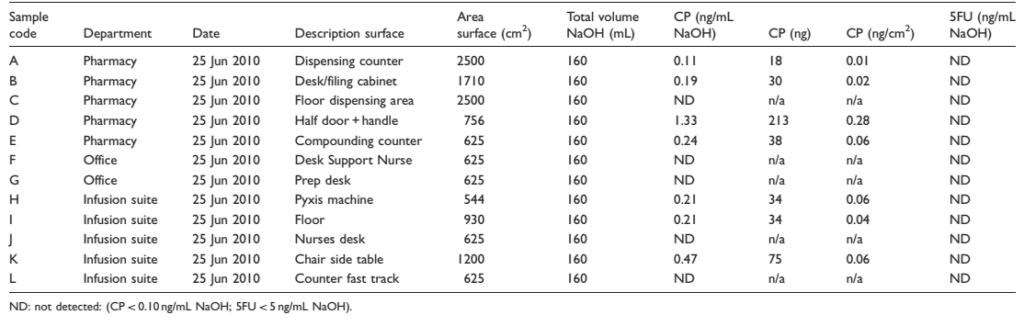
Table 1. Cyclophosphamide (CP) and 5-fluorouracil (5FU) in wipe samples after the use of safety pins and without any prior cleaning (baseline contamination)
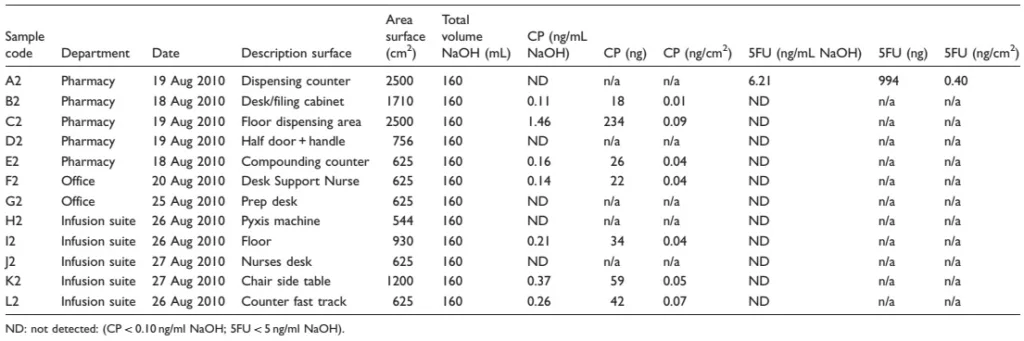
Table 2. Cyclophosphamide (CP) and 5-fluorouracil (5FU) in wipe samples after implementation of the CSTD and after cleaning (start test period)
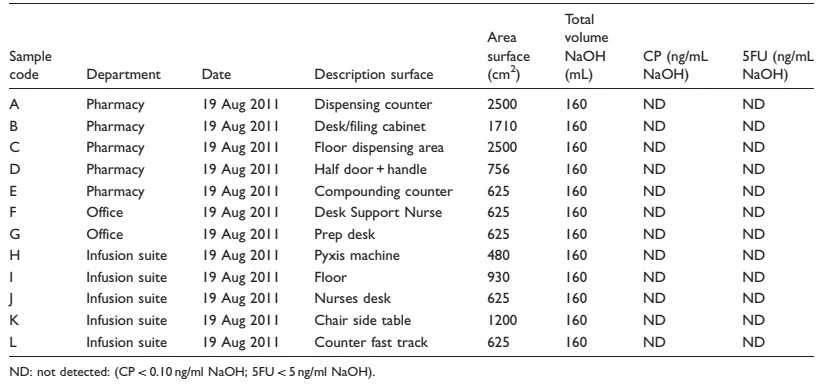
Table 3. Cyclophosphamide (CP) and 5-fluorouracil (5FU) in wipe samples one year after implementation of the CSTD.
Materials and methods
Twelve locations were chosen to be tested for environmental contamination with the cytostatic drugs cyclophosphamide and 5-fluorouracil. The twelve locations included five within the pharmacy, five in the infusion suite area and two in office spaces. The areas tested remained identical throughout the study, with the exception of the automated drug distribution station which was replaced in the first quarter of 2011. Testing sites were determined, measured and the area of each was calculated in square centimeters.
The wipe samples were taken three times. The first samples were obtained on 25 June 2010, the second samples were obtained over the period between 18 and 27 August 2010, and the third samples were obtained on 19 August 2011. All samples were collected by the lead pharmacist at the cancer center. The first samples were collected in June 2010 without prior cleaning to measure the baseline levels of contamination that was occurring with use of the containment technique being used at the time. Implementation of the CSTD occurred concurrently in the pharmacy and the infusion suite in July 2010. Time was allotted for the pharmacy technicians and the nurses to adjust to using the new devices. The pharmacy, infusion suite and offices were cleaned using wipes that contained sodium hypochlorite 0.55% solution. The cleaning was done by a pharmacist and pharmacy technician. The second samples were collected in August 2010 after the implementation of the new devices and the sodium hypochlorite cleaning technique to determine whether the contamination was fully removed. The third samples were collected in August 2011, approximately 1 year after implementation of the devices.
The EquaShieldÕ system24 uses a double membrane for drug transfers to ensure dry connections. The unique syringe is airtight and contains two chambers, the distal chamber for air and the proximal for liquid. Likewise, the connector has two needles to allow for air and liquid exchange. The air contained behind the plunger of the syringe (distal) is transferred into the drug vial when liquid drug is withdrawn into the syringe (proximal).
The wipe samples were taken using Cyto Wipe Kits (Exposure Control Sweden AB). To collect test samples, an aliquot of 0.03 M sodium hydroxide solution from the Cyto Wipe Kits was applied to each target area and wiped off twice with dry tissue paper. The tissue paper was then placed in a plastic container with a screw cap and immediately frozen and stored.
The samples were analysed on a gas chromatography-tandem mass spectrometry method system. Specificity and sensitivity are increased using gas chromatography-tandem mass spectrometry method instead of gas chromatography/mass spectroscopy.25,26
The analysis of 5-fluorouracil was performed on a high-performance liquid chromatography system with ultraviolet detection.10,11
Results
Thirty-six samples were collected throughout the study. The results of the analysis of the wipe samples are presented in the Tables 1–3. The contamination per square centimeter is calculated assuming a 100% recovery and wipe efficiency. Thus, all results are underestimates. The detection limits for the analysis of cyclophosphamide and 5-fluorouracil were 0.10 and 5 ng/mL sodium hydroxide, respectively.
The results from the first two sets show contamination with cyclophosphamide on about half of the positions in all departments during both collection periods (Tables 1 and 2). However, levels of contamination were very low and mostly just above the detection limit of the analytical method. The highest level of contamination was found on the door and handle in the pharmacy. Contamination was found in one of the office spaces upon the second collection. Contamination with 5-fluorouracil was only observed on the dispensing counter in the pharmacy during the second collection period. The results from the final collection period show no contamination with cyclophosphamide or 5-fluorouracil in the pharmacy, infusion suite or offices of the cancer center.
Discussion and conclusion
Exposure to antineoplastic agents is harmful to healthcare workers. Surfaces that are contaminated are touched when healthcare personnel are not using PPE. Sampling of the biological safety cabinet was not done in this study. The outside of vials being used during compounding may be contaminated with cytotoxic agents.27 Our goal was not to show that contamination exists inside the biological safety cabinet but rather to determine whether common areas were more likely to cause exposure, if contaminated, of healthcare personnel.
The initial sampling results showed environmental contamination with cyclophosphamide in several departments. However, the level of contamination was very low compared with historical data.12 Contamination with 5-fluorouracil was only observed at one position. This is probably caused by a higher detection limit for the analysis of 5-fluorouracil compared to cyclophosphamide.
Sodium hypochlorite-containing wipes were used to clean surfaces prior to the second sampling. This is not ideal, as bleach is not effective at removing all antineoplastic agents and the concentration of the wipes was low. However, this is the generally accepted cleaning practice at this institution. It was essential to determine that the CSTD would reduce surface contamination given the chosen cleaning process.
Other studies have shown environmental contamination with cytostatic drugs in pharmacies and administration areas.10–12 The initial results of this study showed very low levels of contamination with cyclophosphamide and 5-fluorouracil compared to the reference data.
The final sampling results, one year after the implementation of the closed-system transfer devices, showed an environment free of contamination from cyclophosphamide and 5-fluorouracil.
At our practice site, nurses must enter the pharmacy to retrieve prepared chemotherapy products for patient administration. The door and handle are touched repeatedly by all personnel, pharmacy and nursing, while not garbed in PPE. Finding the highest level of contamination on this surface was not surprising, but it was confirmation that PPE alone cannot protect healthcare workers from antineoplastic agent exposure.
One of the samples from an office was from a desk of a physician’s support nurse. The employee did not administer chemotherapy nor did the staff member work in the infusion suite. Finding contamination with cyclophosphamide at this location demonstrated that surface contamination could spread throughout a building.
The years of experience and expertise of the pharmacy technicians (a combined 21 years of experience for two technicians) at compounding antineoplastic agents may have been a reason for the low level of contamination initially. In addition, the expertise of the infusion suite nurses (each averaging twenty years of experience) likely contributed to this observed low level of contamination.
Implementation of the closed-system transfer devices for preparing and administering chemotherapy eliminated surface contamination with cytotoxic agents at the ambulatory cancer chemotherapy infusion center.
The NIOSH22 and United States Pharmacopeia’s current USP 79723 standards only recommend the use of CSTDs when preparing and administering chemotherapy. A ‘‘safe’’ level of exposure to antineoplastic agents by healthcare workers is unknown. Based on the positive findings that CSTDs can eliminate surface contamination with antineoplastic agents, guidelines should be adapted to require their use.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors have no conflict of interest to disclose.