Methods: On study day 0, ICG solutions of 2.5mg/mL were prepared in 5mL PP syringes, reconstituted with SWFI. Three syringes were stored at 25°C, 4°C, -20°C or -67°C. ICG concentrations were determined 8 times over each study period at each temperature using a validated
stability indicating analytical method. Chemical stability was based on the intersection of the lower limit of the 95% confidence interval of the observed degradation rate and the time to achieve 90% of the initial
concentration (T-90).
Results: The analytical method separated degradation products from ICG such that the concentration was measured specifically, accurately and reproducibly (1.73% (CV(%)). Analysis of variance revealed significant differences in percent remaining due to study day (p=0.009) and temperature (p=0.035). The calculated T-90, with 95% confidence, exceeded the 28-day study period for syringes stored in the freezer at
either -20°C or -67°C. The calculated T-90, with 95% confidence, was 34.11 hours at 25°C and 37.38 hours at 4°C.
Conclusions: We conclude that 2.5mg/mL solutions of ICG may be stored frozen at -20°C or -67°C for up to 28 days, but at 4°C or 25°C, solutions should be stored for only 36-hours. Syringes stored in the freezer for up 28-days can be withdrawn from the freezer, allowed to thaw, but should be used within 24-hours of withdrawal from the freezer. Under these conditions more than 92.5% of the initial ICG concentration will remain at 24 hours.
Development of an On-Going Sterility Monitoring Program for Single-Use Vials Undergoing Multiple Access Following Application of a Closed System Transfer Device
Charbonneau LF¹, Carating H², Mascioli M¹, Iazzetta J¹, Perks W¹, Stinson J¹, Nedzka-Rajwans I¹, Walker SE²
¹Department of Pharmacy Sunnybrook Health Sciences Centre, Toronto, ON
²Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
Background: Closed system transfer devices (CSTD) are designed to protect healthcare workers from exposure to hazardous drugs. These devices have also been shown to minimize microbial contamination of single use vials (SUV). However, NAPRA has suggested that annual testing of the CSTD is necessary to assure continued sterility. Since validation requires more than 3000 transfers, the feasibility of an on-going monitoring program was investigated.
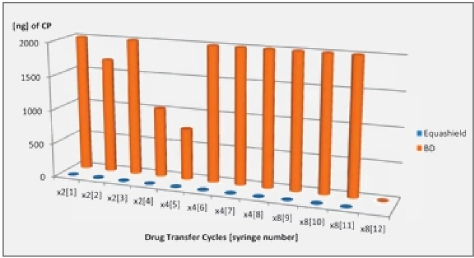
Objective: To test whether attaching a CSTD (Equashield®) to SUVs can minimize microbial contamination and extend the “use-by” date following multiple withdrawals under extreme-use-conditions.
Methods: An Equashield® vial adapter was attached to one 20-mL vial containing sterile TSB growth medium and placed in each of 4 biological safety cabinets weekly. 1-mL samples were drawn from each vial at immediately following application of the CSTD and at 48 and 168 hours. Prior to sample withdrawal vials were visually inspected for turbidity. After 1 week, vials were collected, incubated at 37oC for 14-days and inspected visually every 2 days for evidence of contamination
(turbitidy).
For positive controls, three TSB vials were inoculated with less than 102 of S.epidermidis ATC12228. As a negative control, three unopened vials of TSB were incubated for 14 days. Stopping rules included growth in 2 CSTD vials in fewer than 100 consecutive vials (contamination rate using lower limit of 80%-Confidence Interval (CI) is greater than 0.20%).
Results: All positive control vials demonstrated growth within 48-hours. All negative control vials showed no growth. During the first 20-weeks of monitoring, all CSTD vials (80 vials – 240 transfers) remained sterile following storage at room temperature for 7 days and subsequent
incubation for 14-days. The 95%-CI of the contamination rate is 0.000 to 0.021%.
Conclusions: Attachment of a CSTD to single-use-vials within an ISO-5 environment has the ability to maintain sterility following multiple withdrawals over 7-days.
Stability of 0.04, 0.1, and 0.2 mg/mL Vitamin K (Phytonadione) in 5% Dextrose in Water Solutions Stored in Polyvinyl Chloride Bags at 4°C over 9 Days
Plaetzer WJ¹, Facca N², Smith N³
¹Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
²Department of Pharmacy, London Health Sciences Centre, London, ON
³Department of Pathology and Laboratory Medicine, 3London Health Sciences Centre, London, ON
Background: To our knowledge, no stability data exist for 0.04, 0.1 and 0.2 mg/ml intravenous vitamin K (phytonadione) solutions in 5% dextrose in water (D5W) in polyvinyl chloride (PVC) bags.
Objectives: To test the physical and chemical stability of the 0.04- 0.2 mg/ml vitamin K solutions in D5W over 9 days at 4°C.
Methods: Prepared solutions were stored at 4°C for 9 days, with samples taken daily and frozen pending analysis. Samples from selected days throughout the study were analysed in quintuplicate by gas chromatography/mass spectrometry, with vitamin K-d7 as the internal standard
(ISTD). Vitamin K/ISTD peak area ratios (PARs) were calculated for and compared to those of Day 0 by calculating them as a percentage of the Day 0 value. Mass spectra of Day 9 drug peaks were compared to those of Day 0 peaks to confirm purity. Physical stability was assessed visually daily.
Results: The vitamin K concentration in the preparation of 0.04 mg/ml vitamin K in D5W declined, with the lower 95% confidence limit falling below 90% of the Day 0 value within 45 hours. The lower limits of the 95% confidence intervals of the 0.1 & 0.2 mg/ml vitamin K solutions always remained above 90% of the day 0 values. Day 9 mass spectra of
all solutions were identical to those of Day 0. All solutions remained visually consistent over the 9 days.
Conclusions: These data support 0.1 & 0.2 mg/ml vitamin K solutions in D5W as physically and chemically stable for 9 days, but 0.04 mg/ml vitamin K in D5W was chemically stable for less than 2 days.