The presence of cytotoxic agents in the urine of operators and in their environment has been demonstrated. The pharmacokinetics of the urinary elimination of cyclophosphamide suggests that these drugs are absorbed cutaneously during handling. In the framework of a more general study on the contamination of hospital environment, the present study addresses the possible presence of cytotoxic agents on the plungers of syringes. The report is based on results indicating that the bacterial contamination of a plunger may result in the contamination of the solution being sampled. The study was divided into two phases. The first phase consisted in measuring the contamination of the plungers of eight syringes used for handling cyclophosphamide. Cyclophosphamide was analysed by gas chromatography – mass spectrometry with a detection limit of 0.1ng/ ml. The aim of the second phase was to localize the contamination on the plunger and thus determine the amount of drug that comes into contact with the gloves of the operators. The contamination was quantified by measuring the activity of metastable technetium. The results of the first phase showed that all the plungers were contaminated with cyclophosphamide amounts varying from 3.7 to 445.7 ng. The second phase showed that the infiltration of liquid onto the plunger depended on the solution being sampled. Almost no infiltration was seen with labelled water, but contamination appeared after the first sampling of a cyclophosphamide solution, then increased as a function of the number of times the plunger was pushed in and out. These results indicate that cyclophosphamide solutions infiltrate onto the plungers of syringes. They suggest that the general procedure for handling cytotoxic agents should be modified, and a regular replacement of syringes should be enforced. They also partly explain why the gloves of 50%/90% operators are contaminated after a single preparation. The contamination seems to depend on the type of solution sampled and the number of samplings. Initial investigations by the manufacturer of the syringes had shown that the acid pH of cyclophosphamide solutions may affect the lubricant of the joint. Our study demonstrates that the contamination of plungers is one of the sources of environmental contamination for health workers handling antineoplastic agents, even in the absence of manipulation errors. More generally, these results demonstrate that the exposure of operators cannot be clearly described unless all existing sources of contamination in their environment are identified. The implementation of suitable procedures should thus take into account all possible sources of contamination, including technical facilities such as the use of a safety cabinet or an isolator.
J Oncol Pharm
Practice (2005) 11: 1-5.
Key words: contamination; cytotoxic; exposure;
syringe plungers
Introduction
In 1979, Falck et al. suggested the possibility that health workers involved in the preparation and manipulation of anticancer drugs underwent occupational exposure to cytotoxic agents.1 The authors subsequently confirmed and quantified such exposure, mainly by measuring agents such as cyclophosphamide in urine. They obtained positive results, then extended their study to environmental contamination.2-7 They showed that the gloves and the overall working environment of these personnel were frequently contaminated by varying concentrations of cytotoxic agents.5,6 The present study, conducted within the framework of a larger study on hospital contamination, focused on the possible contamination of syringe plungers by the solution being sampled, on the basis that the bacterial contamination of syringe plungers can lead to the contamination of the solution itself.8 The presence of a cytotoxic agent on the plungers is a possible source of environmental contamination for people handling the drug whose gloves are generally contaminated, even when no manipulation error is made
MATERIALS AND METHODS
This study was conducted at the Centre Le´on Be´rard (France) with 50-ml, three-piece Becton Dickinson syringes. These syringes were chosen because of their long plungers that compel operators to touch them with their gloved hands. The study consisted of two phases.
Phase 1
In order to study the actual contamination of syringe plungers employed for the preparation of cyclophosphamide solutions, eight syringes were used for about 8 h (9:00 – 17:00), then samples were taken throughout the day when the syringes were needed to fill prescriptions. The number of times the plunger was pushed in and out was recorded. At the end of the day, a half compress (20*20, Tetra Medical) impregnated with 5 mL of water for injectable preparations was applied onto the polypropylene plunger after it had been pulled out to its fullest extension. The compress was then stored in a glass flask at -20oC until analysis.
Sample treatment. The compress was placed in a silanized glass tube with 0.1 mL of a solution of 250 ng/mL trofosfamide (internal control) and 0.5 mL Tris buffer, pH 8. The cyclophosphamide was extracted with 15 mL of unstabilized diethyl ether. The sample was shaken mechanically for 10 min, then the organic phase was removed, centrifuged at 3000 rpm for 6 min, then placed in a silanized glass tube. The aqueous solution was extracted again as before. The entire organic phase was dried with anhydrous sodium sulfate, then evaporated under a light stream of nitrogen at 35oC until a volume of 2 mL was obtained. The diethyl ether residue was transferred to a 3-mL glass flask, then evaporated to dryness under a light stream of nitrogen at 35oC.
Derivatization. The dried residue was treated with 100 mL of ethyl acetate and 100 mL of trifluoroacetic anhydride (derivatization agent). The solution was shaken for a few seconds, then heated at 708C for 15 min. After the solution had been returned to room temperature, it was evaporated to dryness under a light stream of nitrogen, then 100 mL of toluene were added. After 5 min mechanical shaking, 1 mL of the solution was injected into the chromatograph.
In these conditions, the mean recovery rate (9/SD) of cyclophosphamide with the sampling method described above was 859/10%.
Analytical conditions. Cyclophosphamide was analysed by gas chromatography-mass spectrometry (GC-MS) with a detection limit of 0.1 ng/mL. We used a Hewlett-Packard 5 MS capillary chromatographic column with an internal diameter of 0.25 mm, a film thickness of 0.25 mm, and a length of 30 m. The carrier gas was helium 5.5, the pressure at the head of the column was 17 kPa, the gas flow was 50 mL/min, and the column flow was about 1 mL/min. The splitless injection mode was used.
Gas chromatographic conditions. The initial oven temperature was 1108C. After 1 min, it was progressively increased by 158C/min to 2808C. After 0.5 min, it was increased by 258C/min to 3108C. After 3.57 min, the oven temperature was decreased to 1108C for 0.2 min before the next injection.
Mass spectrometry. The interface and source temperatures were 2808C and 2008C, respectively. The energy of the ionizing electrons was 70 eV, and the trap current was 150 mA.
Characteristics of selected ion monitoring. Two entry windows were used: the first one from 9.00 to 11.20 min, during which the mass filter was adjusted to ions 307, 309 and 212 of cyclophosphamide, and the second one from 11.20 to 13.00 min, during which the mass filter was adjusted to ions 273, 275 and 182 of the internal standard. Under these conditions, cyclophosphamide trifluoroacetate and trofosfamide were eluted at retention times of 10.308 and 12.080 min, respectively.
Phase 2
The objective of the second phase was to localize the contamination on the plunger with solutions of technetium-99m, in order to determine what quantity of cytotoxic agent could come into contact with the gloves of operators. Two solutions were prepared:
- 50 mL of a solution of 99mTc, with an activity of 1 GBq;
- 50 ml of a solution of 20 mg/mL cyclophosphamide in water with 1 GBq of 99mTc.
Both were placed in 50-mL polyvinyl chloride bags. Three tests were performed.
- In the first and second tests, 1, 3, 5 and 10 samples of the solution of 99mTc and of the solution of cyclophosphamide and 99mTc were drawn up by an operator who avoided touching the plunger with his gloves during sampling. The axis of the plunger was unchanged.
- In the third test, 1, 3, 5 and 10 samples of the solution of cyclophosphamide and 99mTc were drawn up by an operator who touched the plunger with his gloves during sampling. The axis of the plunger was thus modified, which corresponds to the actual situation in normal use. After each in-and-out movement of the plunger, the gloves were removed and the contaminating activity measured with an external Canberra probe. The data points reported correspond to the mean of activities measured on four different syringes.
Sampling on plungers. Three samples were taken from the plunger of each syringe with swabs impregnated with double-distilled water (Figure 1). Samples corresponded to the surface of the upper half of the plunger (E1), the surface of the plunger adjacent to the joint (E2) and the surface of the joint itself (E3), respectively
Analytical method. Activity was measured using a Packard Cobra counter with five measurement wells. Both the background activity and the rate of decay of 99mTc were taken into account in the measurements.
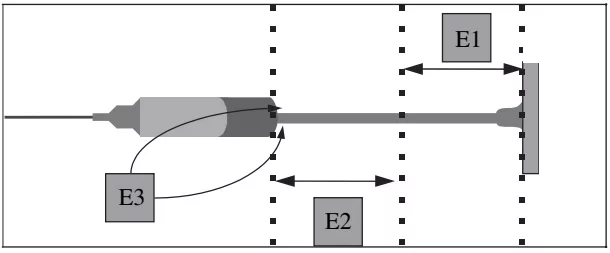
Figure 1. Location of samples from syringe plungers.
RESULTS
Phase 1
The plungers of the eight syringes tested were contaminated with cyclophosphamide (Table 1) (mean value, 71.5 ng; range, 3.7-445.7 ng). Cyclophosphamide concentration in the solution was 20 mg/mL, which corresponds to a mean volume of 3.6 nL (0.2-22.3 nL).
Contamination reached 50 ng or more in one of three syringes, and about 5 ng in two of three syringes. No relationship was found between the number of in-and-out movements of the plunger and the quantity of cyclophosphamide on the plunger.
Phase 2
The results of the second phase are shown in Tables 2 and 3. Almost no contamination was found when labelled water was used (A). Contamination remained under 1 nL, even after 10 in-and-out pushes, although a slight increase was noted when the number of plunges increased. The contamination of the plungers was consistently greater with the solution of radiolabelled cyclophosphamide than with the pure radiolabelled solution, regardless of the test or the number of in-and-out pushes. This difference became obvious after the first use of the syringe, whether the operator touched the plunger with gloves or not; however, the total contamination of the plungers was more important after the operator had touched the plunger than otherwise, but this difference disappeared after 10 plunges.
Upper and lower surfaces of the plungers (E1 and E2). The contamination of the upper and lower surfaces of the plungers corresponds to the amount of contaminant that could come into contact with the gloves of operators.
- Almost none (90 pL, Table 2) was found with radiolabelled water, regardless of the number of inand-out plunges.
- Contamination increased after only five in-and-out plunges in the test with no contact with the plunger. Little contamination was seen after the first in-and-out plunge, but the amount increased rapidly as a function of the number of plunges; a 50-fold increase was noted between one and 10 plunges (from 0.08 to 3.99 nL).
DISCUSSION
Our results show that cyclophosphamide infiltrates onto the plungers of syringes, suggesting that the general procedure for the manipulation of cytotoxic agents should be modified. Syringes should not be used throughout the day, but should often be replaced with new ones. Systematic replacement after each manipulation is not justified, as we have shown that leakage onto the plunger occurs only after a syringe has been used several times.
These results also call into question the use of twopiece syringes for reconstituting antineoplastic drugs, as these syringes are less watertight than three-part syringes. This study may lead, as was the case for gloves, to establishing recommendations for the use of certain syringes for the manipulation of cytotoxic agents.
The infiltration onto the plunger is higher with the cyclophosphamide solution than with labelled water, and the quantity increases with the number of uses of the syringe. We suppose that the cyclophosphamide solution itself reacts with the joint or the syringe to ease its way onto the plunger. Initial investigations have shown that the acid pH of the cyclophosphamide solution may affect the silicone used to lubricate the syringe.
The finding that cyclophosphamide infiltrates onto the plungers of syringes further accounts for the contamination of gloves, as well as flasks, during drug manipulation,5,6 even when no handling error is made. The different amounts deposited on the upper and lower surfaces of the plunger in the various tests (either when operators touched the plunger on sampling cyclophosphamide or when they did not) indicate that up to 10.2-53.4 ng of the drug may contaminate the gloves of operators after 5-10 in-and-out plunges (Table 3). This contamination, when repeated all day and going unrecognized, or when not efficiently dealt with, might contribute to the occupational exposure of operators.
Table 1. Amounts and volumes of cyclophosphamide on the plungers of the eight syringes
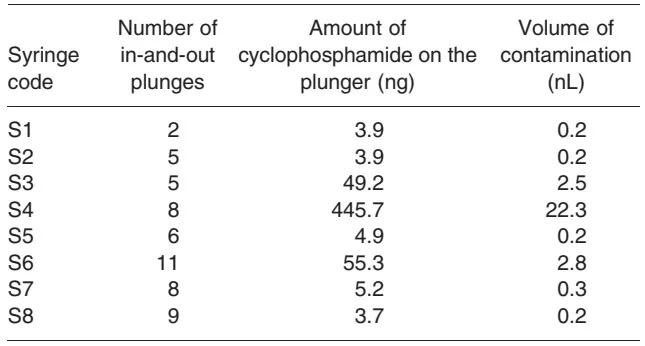
Table 2. Volumes (nL) of contaminating agents on the plungers of syringes; results of three tests
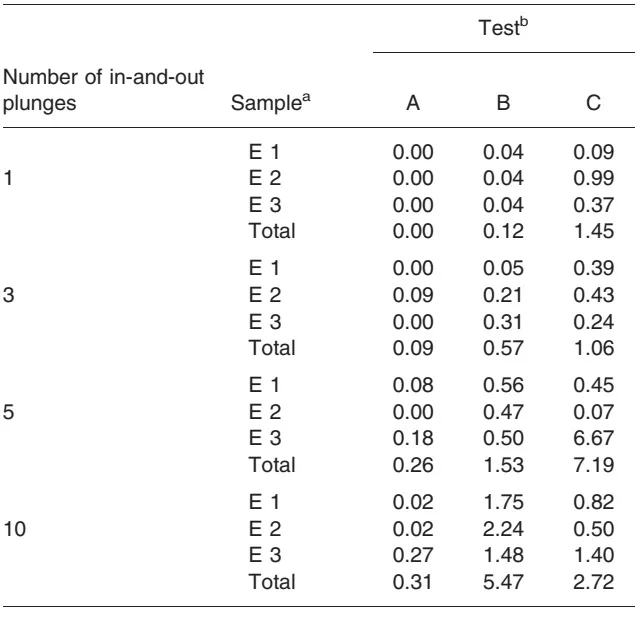
aE1, upper surface; E2, lower surface; E3, joint.
bA, sampling of 99mTc solution without touching plunger; bB, sampling of a
solution of cyclophosphamide and 99mTc without touching plunger; bC,
sampling of a solution of cyclophosphamide and 99mTc when touching
plunger.
- No such trend was found in the third test, when the operator touched the plunger (C). The contamination remained relatively stable, with volumes on upper and lower surfaces varying between 0.52 and 1.32 nL (Table 2). However, the contamination after one and three plunges was, respectively, 13.5 and 3.2 times greater in this test than when the operator did not touch the plunger (B). On the opposite, it was, respectively, 2.0 and 3.0 times lower than in B after 5 and 10 plunges.
Surface of the joint (E3). As for upper and lower parts of the plunger, the contamination of the joint was negligible in the test with 99mTc only, although it slightly increased with the number of in-and-out plunges. A linear progression of the joint contamination was seen in the test with cyclophosphamide when the manipulator did not touch the plunger (B). This was not the case when the plunger was touched (C): wide variations were found in the amount of contamination (0.24-6.67 nL), regardless of the number of in-and-out plunges. Changing the axis of the plunger therefore appears to play a critical role in the contamination of the joint.
Table 3. Amounts (ng) of cyclophosphamide present on plungers; results of two tests
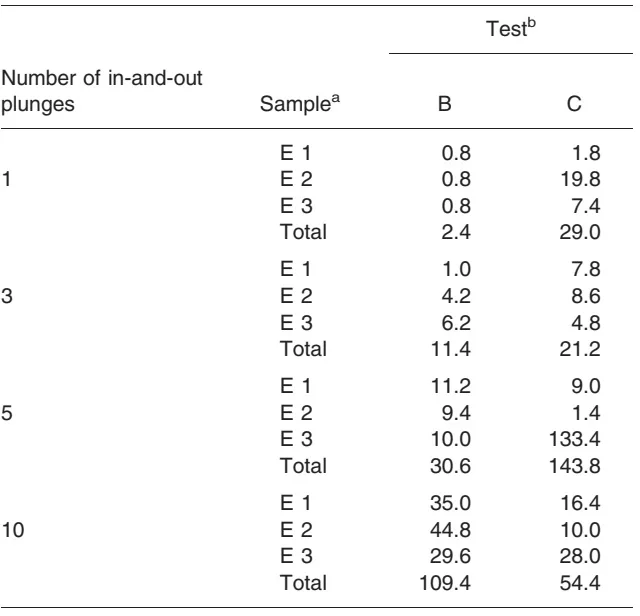
aE1 upper surface; E2, lower surface; E3, joint. bB, sampling of a solution of cyclophosphamide and 99mTc without touching plunger; bC, sampling of a solution of cyclophosphamide and 99mTc when touching plunger.