1. BACKGROUND INFORMATION AND RATIONALE
1.1 Background
There are several routes of exposure to hazardous drugs (HD) throughout the drug-handling chain, from pharmaceutical compounding to patient dose administration. Closed System transfer devices (CSTD) are used to protect against such worker exposures to HDs. CSTDs should have the ability to function as a closed system that restricts drug mass (vapor or liquid) from crossing the system boundary and escaping into the surrounding environment [1,7]. However, in the absence of CSTD performance standards that currently apply to drug containment and given uncertain claims of protective performance, the protective efficiency of certain CSTDs remains in doubt for at least two of these routes of exposure.
- The first route of exposure occurs during the reconstitution of HDs when the diluent injection forces the venting of air saturated with drug vapor from the vial headspace to the environment. This is applicable only to vented CSTD models, the “air-cleaning systems,” designed to filter and remove aerosol or vapor contaminants from airstreams that might escape the drug transfer system [1,7]. Air-Cleaning CSTDs came to market after “barrier CSTDs,” offering significant cost savings. However, their efficacy in preventing the escape of drug vapor remains under heavy scrutiny to this date.
- The second route of exposure occurs in a syringe during and after injection of HDs, when a layer of the drug may remain due to adhesion on the inner walls of regular syringes used with air-cleaning and barrier-type CSTDs [12,13,14], Layers of drug compounds with vapor generating potential may evaporate into the environment and potentially contaminate the syringe plunger. This is applicable to all CSTDs that use regular open barrel off-the-shelf syringes.
In the absence of such worker protection standards, the users (e.g., health care facilities and pharmacies) have no worker-protection performance basis upon which to make their selection of a CSTD, and they may be inclined to select a product based solely upon acquisition costs and uncertain claims of protective performance [1]. Since 2015, The National Institute for Occupational Safety and Health (NIOSH) has been developing performance test protocols for CSTDs. NIOSH’s first “Vapor Containment, Performance Protocol” introduction in 2015 used IPA alcohol as the surrogate [1]. NIOSH continues to adhere to detecting escaped vapor concentrations of drug surrogates as the basic testing approach. In 2016 NIOSH introduced 9 surrogates (later extended to 1 1 surrogates) to be used in the “Performance Test Protocol for CSTDs [7].” While the testing methods and equipment have significantly changed over the years, these surrogates remain under current consideration, including alcohols for testing barrier-type CSTDs (NIOSH 2019) [8]. NIOSH’s surrogate selection strategy involved the selection of Thiotepa, the HD with the highest known vapor pressure as a worst-case scenario. To build in a safety factor, surrogates were considered with vapor pressures in the range, starting with Thiotepa and going up to approximately 100 times that [7], NIOSH also stated in its 2015 protocol that “A CSTD design that relies upon aerosol filtration to clean air that exits the drug transfer system is worker protective only if use of the CSTD is limited to compounding drugs that have no vapor generating potential. For drug compounds with either known or uncertain vapor generating potential, the protective selection of air-cleaning CSTDs requires specific test data for every’ drug type and formulation they will contact, since air-cleaning technologies can have varying efficiencies based upon the chemical and physical make-up of the contaminant.”[1] This study evaluates two out of nine NIOSH surrogates for their suitability as challenge agents for testing the aforementioned two routes of exposure. The evaluation shall determine whether CSTD efficiencies vary upon the chemical and physical make-up of surrogates to the degree that inefficient surrogates, including alcohol, shall be excluded or used only in combination with efficient surrogates.
1.2 Study Objectives
The purpose of the study in its first stage was to determine that each vented air-cleaning CSTD can have varying vapor containment efficiencies with varying contaminants since some vented CSTDs could contain vapor of one type of contaminants and absolutely fail with others.
In its second stage, the purpose of the study was to establish scientific evidence for vapor release from regular open-barrel syringes as a route of exposure and further to determine that varying contaminants can cause varying levels of vapor contamination from open-barrel syringes, ranging from high contamination to none.
This study tests four commercially available vented CSTDs using two out of nine hazardous drug surrogates selected by NIOSH for testing air-cleaning CSTDs and uses NIOSH equipment, thereby identifying ineffective surrogates and their alike that should be excluded from the NIOSH list of surrogates. Ineffective surrogates may lead users to make their selection of CSTDs that have no worker-protection performance.
1.3 Introduction
This was a three stages study:
- In the first stage, air was sampled by an FTIR analyzer directly in front of the vent opening of a CSTD. The analyzer detects and quantifies in real-time the vapor concentrations that are vented out of the CSTD during the injection of 50ml of water into a vial containing 3ml of surrogate. This process is identical to reconstitution.
- In the second stage, 50ml of the surrogate/water solution from the first stage was transferred to an IV bag using the same CSTD and syringe. Air was sampled by the analyzer from the open back of the syringe during the injection of the surrogate solution into the IV bag. This process is identical to standard hazardous drug transfer from vial to bag using CSTD.
- In the third stage, surrogate incompatibilities with CSTDs were excluded. All tested devices were stored for at least 72 hours, and their mechanical integrity and functionality were examined.
The first stage complies with recent NIOSH’s approach to separately test the air-cleaning feature. In its 2019 update [8], NIOSH suggested a two-stage approach to testing Air Cleaning CSTDs: “Stage 1-Air Filter Test: The CSTD via! adapter of an air-cleaning CSTD was first to he evaluated” [8];
NIOSH also suggested in its 2019 update, the use of Gasmet DX4040 FTIR analyzer for testing barrier type CSTDs with Ethanol (see test layout in Fig. 1) [8];
However, the universal DX4040 analyzer is designed to detect hundreds of various gases with very low limits of detection, including five of the nine NIOSH surrogates. This analyzer is ideal for this study as it allows for straightforward testing in real-time for vapor containment efficacy of both the Air-Cleaning feature of vented CSTDs and vapor that may be generated by regular open barrel syringes used with CSTDs for compounding and administration of hazardous drugs. This study is based on characteristics of handling Cyclophosphamide, Ifosfamide, Cytarabine and similar drugs using the same reconstitution and transfer procedures and range of dosages or concentrations [Package Inserts 9, 10, 11]. NIOSH selected its surrogates to represent undiluted Active Pharmaceutical Ingredients (API) [7], which Ifosfamide is an example. Ifosfamide is a common hazardous antineoplastic drug listed on the NIOSH List of Hazardous Drugs. Each vial contains 3 grams of Ifosfamide which is free of any excipients. Injection of diluent (e.g., SWFI) into Ifosfamide vial is required for reconstitution [Package Insert 9]. In all terms, the testing represents the actual usage of drugs and CSTDs. This study doesn’t aim to provide absolute vapor quantities, but the performance metric of interest as the maximum concentration value (in ppm) observed during each test run. 1
1.4 Methods
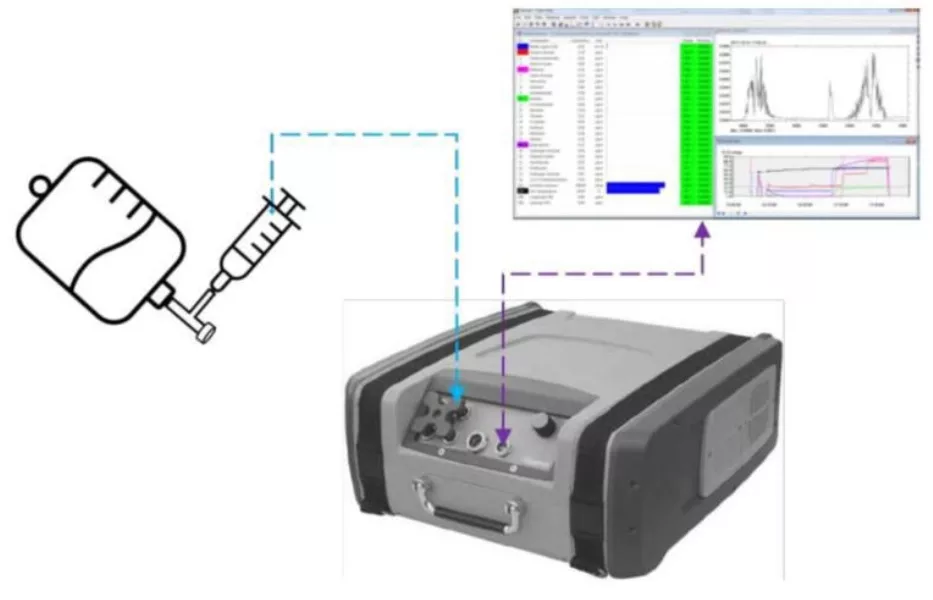
The methods of testing are illustrated in Figures 2 and 3, which are based upon the NIOSH approach (Fig. 1) but simplified. Figure 4 illustrates the first stage, the “Air Filter Test: The CSTD vial adapter of an air-cleaning CSTD was to first be evaluated” (NIOSH 2019) [8].
The Gasmet FTIR analyzer has a flexible air sampling tube with a 60mm diameter glass funnel attached on its end. The funnel allows for efficient air sample suction since vented gases from CSTDs may spread in all directions.
Similar to the NIOSH test layout (Fig. 1), the analyzer has attached to its air-outlet another flexible tube with a 50mm glass funnel on its end to create a loop and significantly increase sample collection efficiency. The openings of both funnels are brought in proximity and face each other. A stand with clamps is used to fixate the assembly in position.
Figure 3 illustrates the second stage: The Gasmet FTIR analyzer has a flexible tube to allow air sampling from the open syringe barrel. For simplicity, the air-outlet is not looped.
Testing methods for the first stage (filter test) are as follows:
- 100mL vials are filled with 3mL of NIOSH surrogate
- CSTD adapters are attached to an IV bag containing sterile water for injection (SWFI)
- CSTD syringe adapters are connected to regular syringes and then are connected to IV bag. The syringes are filled with 50mL of SWFI in accordance to the individual manufacturer IFU.
- CSTD vial adapter is attached to the surrogate vial, and the CSTD syringe is attached to said vial adapter.
- The vent opening of the vial adapter is placed between the two funnels, and 50mL of SWFI is injected within 12 seconds into the vial, thereby monitoring the real-time analysis data on screen.
- The analyzer runs on continuous mode and collects the vented air from CSTDs. The results are displayed every second in ppm and indicate whether or not the Air-Cleaning system is able to filter out the surrogate gas and vapor component from the vented air.
- The above steps were repeated with each of the four CSTD brands 10 times and with each of the two surrogates (4 x 10 x 2 = 80 replicates).
Testing methods for the second stage (syringe test) are as follows:
- 50mL of the surrogate solution is withdrawn into a syringe from the vial that was tested in the first stage, and the syringe is then connected to the IV bag for injection.
- The end of the sampling tube is placed in the syringe barrel’s back opening, and 50mL of the surrogate solution is injected into the IV bag, thereby monitoring the real-time analysis data on the screen.
- The analyzer runs on continuous mode and collects the vapor generated from the syringe. The results are displayed every second in ppm and indicate whether or not surrogates stick to the syringe inner walls exposed to the environment and generate vapor.
- The above steps are repeated with each of the CSTD syringes from the first stage (80 replicates).
- Positive and negative controls are performed for both first stages.
- 50mL of the surrogate solution is withdrawn into a syringe from the vial that was tested in the first stage, and the syringe is then connected to the IV bag for injection.
- The end of the sampling tube is placed in the syringe barrel’s back opening, and 50mL of the surrogate solution is injected into the IV bag, thereby monitoring the real-time analysis data on the screen.
- The analyzer runs on continuous mode and collects the vapor generated from the syringe. The results are displayed every second in ppm and indicate whether or not surrogates stick to the syringe inner walls exposed to the environment and generate vapor.
- The above steps are repeated with each of the CSTD syringes from the first stage (80 replicates).
- Positive and negative controls are performed for both first stages.
Testing methods for the third stage (surrogate compatibility validation) are as follows
- All tested devices after the first and second stage are collected and stored at room temperature for the duration of 72 hours.
- After 72 hours, the CSTD devices are checked for mechanical and visual integrity, leaks, functionality and filter blockage.
2 Description of Investigational Products
2.1 Devices
This study includes the testing of four commercially available CSTD products and a commonly used regular syringe:
2.2 Surrogates
Two surrogates for hazardous drugs were utilized to assess vapor containment. The surrogates were chosen based on preliminary testing and study development:
3 Data Collection
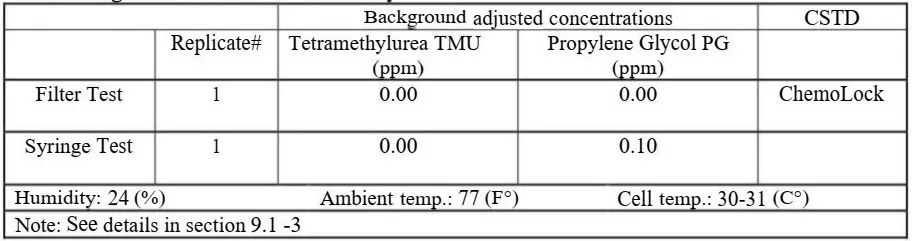
For purposes of the Vapor Containment Efficacy protocol, the performance metric of interest is the maximum value observed during each test run. In order to create Background (BG) adjusted test data, the maximal BG concentration must be subtracted from the maximum concentration data point (value) collected during the test run. Maximum BG concentration data shall be observed and recorded for at least five seconds prior to the start of each test. As part of the data analysis, the maximum observed BG concentration shall be then subtracted from the maximum concentration value observed during the test run to create BG adjusted test data. The final test result equals the BG adjusted maximum concentration [1], “Within the environmental sciences, where environmental data are evaluated to estimate true exposures, the rules for handling below LOD data can he complex and labor intensive. For purposes of the CSTD evaluation protocol, the performance metric of interest is the maximum value observed during the test run.”[1]
4 Testing Conditions
- No direct airflow or turbulences on testing site
- Ambient temperature 72°F – 77°F
- Keeping all materials and equipment at ambient temperature
- Regularly refreshing the room air
- Keeping undiluted vials upright to avoid contact to vial stopper
5 Study Setup
- Analyzer and laptop were wired to operate in cable mode and powered. The CalcmetPro software that operates the analyzer is specified by Gasmet and was used with the laptop.
- Following at least 45 minutes of analyzer warm-up, the zero calibration for the background was performed at the beginning of every day of testing using a nitrogen gas of 99.999% purity in accordance with the analyzer manufacturer’s instructions.
- After the calibration, the “analysis time” in Calcmet software was set to “continuous” mode, “1 second measuring time” and “pump enabled.” This setup allows for continuous air sampling (suction) with recurring 1-second-long cycles of analysis (updated results shown every second).
- The sampling tube was connected to the “sample in” port and the return air tube connected to the “sample out” port. Glass funnels were attached to the ends of the tubes and clamped to the testing stand inclined at a 45° angle (see configuration in Fig. 2),
- Empty 100mL vials were filled with 3ml of Propylene Glycol using a pipettor and were sealed with a rubber stopper and aluminum cap using the crimper device. Vials were labeled.
- The same fill process was repeated with Tetramethylurea using a new pipette.
- CSTD bag spike adapters were attached to SWFI bags, and the respective CSTD syringe connectors were attached to syringes. All syringes with the respective four types of CSTDs attached were accurately filled with 50mL of SWFI in accordance with the CSTD manufacturer’s instructions for use.
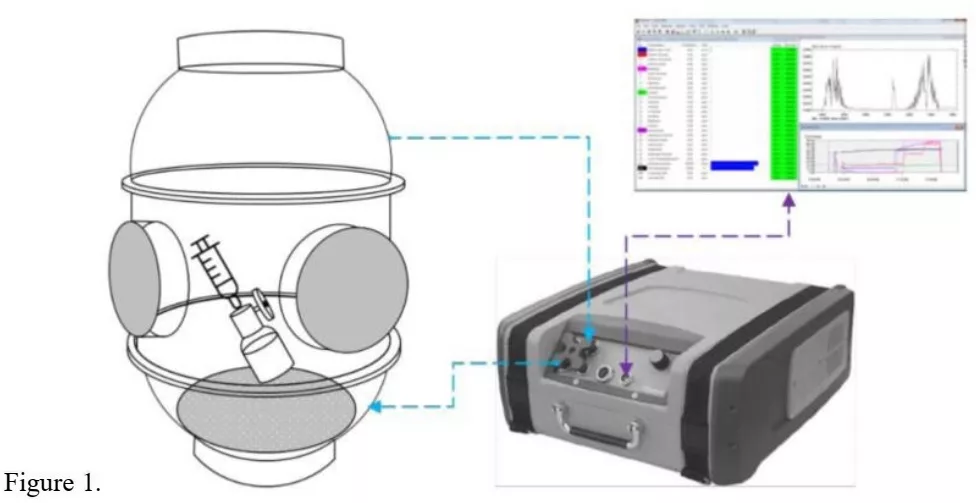
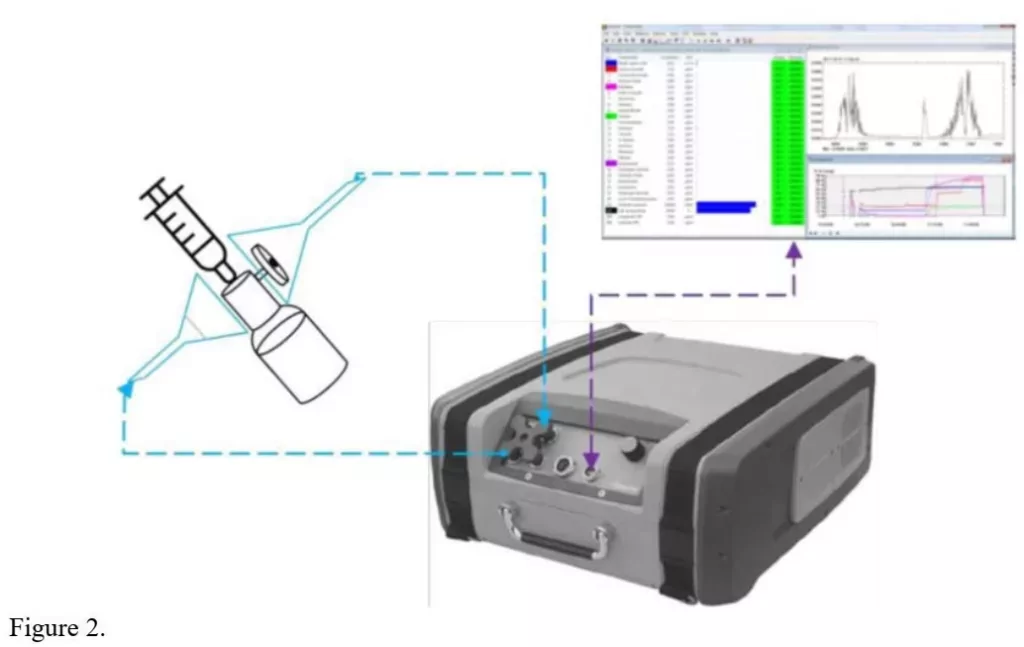
Figure 3:
2.1 Device
2.2 Surrogates

13 Results
13.1 Final Test Results
1. OnGuard/Tevadaptor CSTD / Gasmet analyzer# 1
2. OnGuard/Tevadaptor CSTD / Gasmet analyzer# 2
3. Chemfort CSr “D / Gasmet analyzer# 1
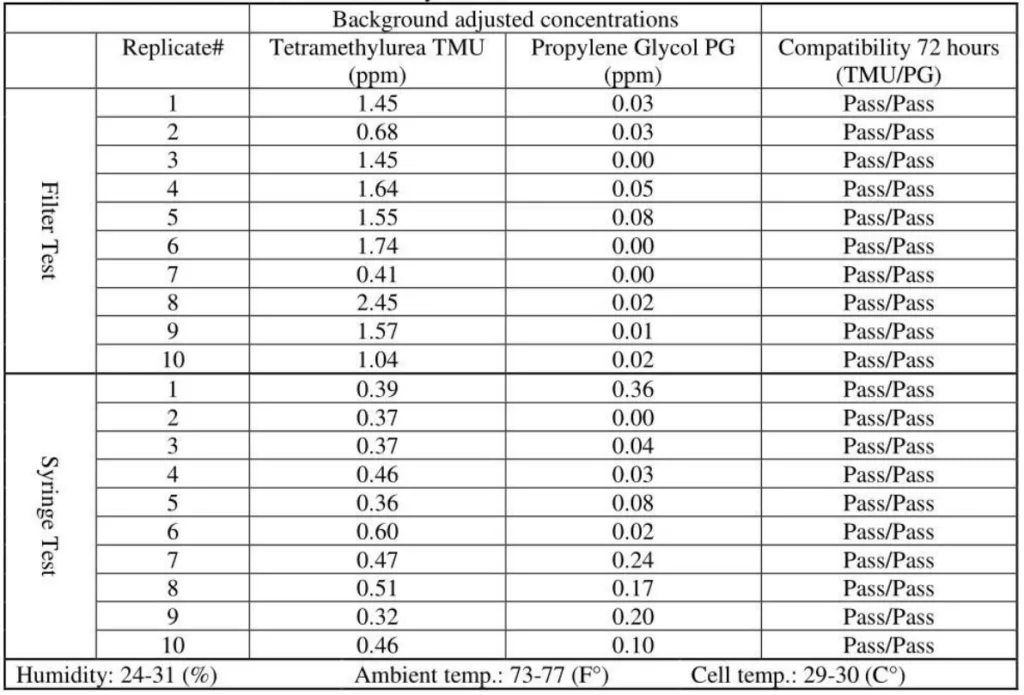
4. SmartSite/Texium CSTD / Gasmet analyzer# 1
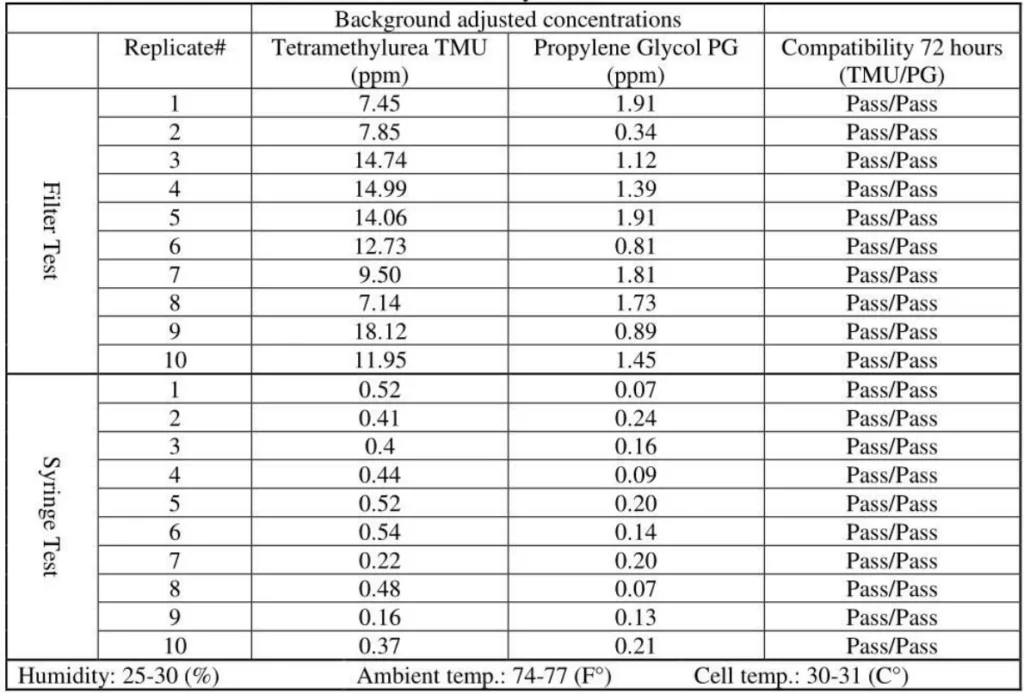
5. ChemoLock CSTD / Gasmet analyzer# 1
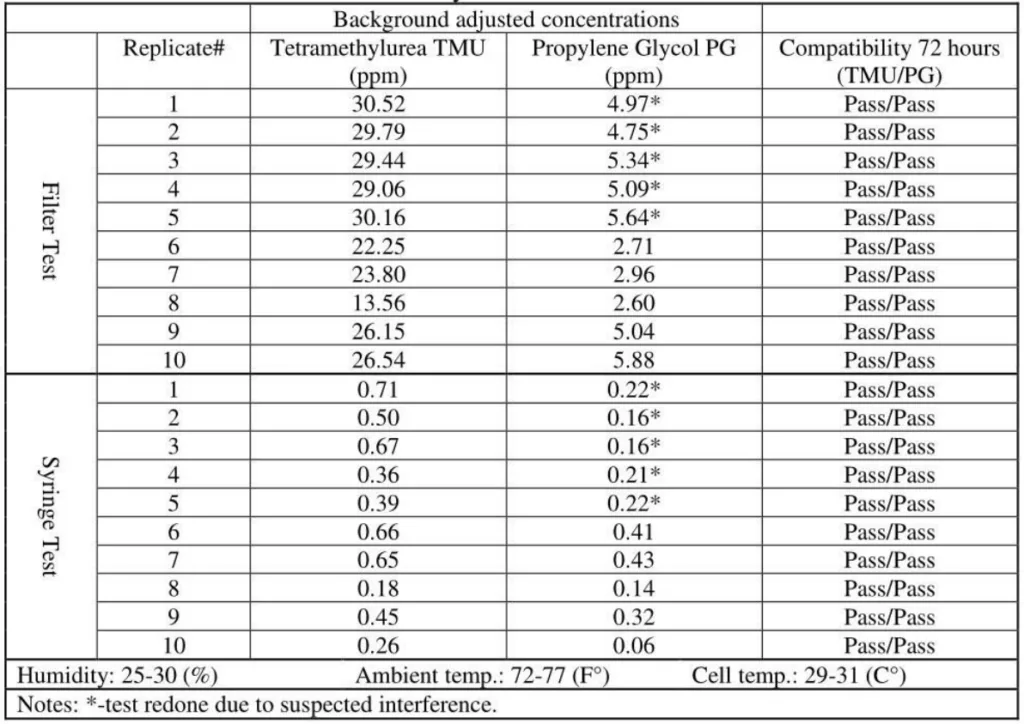
6. Positive Control / Gasmet analyzer# 1
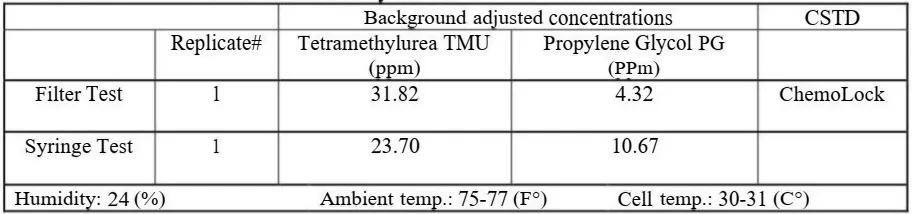
7. Negative Control / Gasmet analyzer# 1
8. 5 seconds Measuring Time Setup / OnGuard/Tevadaptor CSTD / Gasmet analyzer# 1
6 Test Procedure First Stage
- Each vial was laid on its side and rolled slowly on the table surface to coat the inner glass walls with the surrogate; this simulates the drug powder that is typically distributed all over the inner walls of the drug vial. The vial was partially inverted while rolling to coat the inner vial neck, thereby avoiding contact between the surrogate and the rubber stopper.
- The CSTD vial adapter was attached to the vial in accordance with the CSTD manufacturer’s instructions for use. For OnGuard/Tevadaptor and Chemfort CSTDs only, the side of the hidden vent opening was visually identified and marked. The syringe previously filled with 50mL SWFI (with its CSTD connector) was connected to the surrogate vial CSTD adapter.
- The connected syringe and vial were clamped in the testing stand with a 45° angle and the vent opening facing up (see Fig. 2). The upper sampling funnel was brought in the closest position to the vent opening of the CSTD vial adapter. The lower funnel was brought in the opposite position at the vial adapter’s underside while facing the upper funnel head-on. The lower funnel was preferred to be a Buchner type because of its better laminar air distribution.
- While preparing for injecting SWFI, all sampled vapor and gases concentrations (also called background concentrations) are displayed on the laptop monitor in ppm every second. Prior to the start of each test run (injection), these background (BG) concentrations were observed for at least five seconds and the maximum BG concentration value was recorded in the data collection form.Note, when analyzing the final test results, the recorded maximum BG concentration must be subtracted from the maximum concentration value (data point) collected during the test run to create BG-adjusted test data. If BG was zero, then there is no need for BG adjustment.
- The SWFI was injected into the vial within 12 seconds by pushing the plunger at a steady pace while observing the laptop monitor for surrogate concentration values to change. After waiting a while for the highest concentration value, it was recorded in the data collection form. Typically, after reaching the highest value, the vapor dissolves in the ambiance and the values begin to decrease until reaching BG levels or zero. In case no vapor was detected, the values remain at BG levels or zero. To accelerate the air cleaning and refreshment in the analyzer for the next test run, one of the funnels was moved aside until the analyzer reading drops to BG levels or zero before starting the next test.
- The ambient temperature and the analyzer’s cell temperature were recorded during the testing.
- In addition to manual recording in data collection forms, the Calcmet software automatically saves all analysis data and spectra. These study files were saved.
- The syringe and vial were unclamped from the stand, disconnected from each other and stored for use in the second stage of the study. Vials were kept upright.
- This testing procedure was repeated for a total of 10 times with the same CSTD type and same surrogate and further repeated with four CSTDs and two surrogates, generating a total of 80 replicates for the first stage of the study.
7 Test Procedure Second Stage
- The clamps, stand, and funnels used in the first stage of the study were removed from the analyzer.
- Only the assembly, as illustrated in Fig. 3 was kept for the second stage of the study.
- The syringe with its CSTD connector was attached to the original vial containing the surrogate solution from the first stage of the study. The vial was inverted, and 50mL of the surrogate solution was drawn into the syringe. The syringe was disconnected from the vial and connected to the original IV bag from the first stage of the study. All steps were performed in accordance with the CSTD manufacturer’s instructions for use.
- The bag and syringe were brought into position as illustrated in Fig. 3 and the sampling tube of the analyzer was placed inside the syringe opening, as shown in Fig. 3.
- Before injecting the surrogate solution into the bag, BG concentrations were observed for at least five seconds and the maximum BG concentration value was recorded in the data collection form.
- The surrogate solution was injected into the bag by pushing the plunger at a normal pace and the sampling tube was allowed to follow the plunger into the syringe barrel.
- After waiting a while for the highest concentration value, it was recorded in the data collection form. In case no vapor was detected the values remain at BG levels or zero. The tube was removed, and the concentration values decrease down to BG levels or zero.
- The syringe with its CSTD connector was disconnected from the bag CSTD adapter.
- This testing procedure was repeated for all 80 replicates from the first stage of the study.
8 Test Procedure Third Stage (Compatibility Validation)
- All test replicates from the first and second stages (syringe, bag, vial, CSTDs) were stored at room
- temperature for the duration of at least 72 hours. Vials were kept in an upright position.
- After 72 hours of storage, the syringes and vials were connected. The plunger was pulled to draw 50ml of air and pushed to reinject. The syringe was disconnected from the vial and connected to the bag. The solution was pulled and pushed from and into the bag. The devices were observed for leaks, functionality and mechanical integrity.
- Visual inspection for damages was performed for all devices, followed by visual inspection for the vent filters of the CSTDs. ChemoClave and SmartSite filters are clearly visible. For accessing the OnGuard and Chemfort filters, the snap-fitted covers were removed. Removing the covers causes absolutely no damage and exposes both filters (hydrophobic and activated charcoal).
- The inspection results were recorded.
9 Study Controls
9.1 Negative Control Procedure
- A negative control test was performed with one CSTD prior to testing with surrogates.
- The negative control is identical to the test procedure with sunogates but with water only.
- An additional negative control is provided by the analyzer during each test run of the study. The analyzer is set to detect both surrogates simultaneously (can detect 25 gases at the same time) and during the injection of one surrogate, the concentration values of the second surrogate remain unaffected, which is a negative control indication.
9.2 Positive Control Procedure
The positive control test was performed by bypassing the CSTD vent filter.
- A representative CSTD with a visible filter was chosen, and using a needle, and a hole was punctured in the middle, through the vent hole, to bypass the filter.
- Regular test procedures with both surrogates were performed and the results of the unfiltered stream of vapor were recorded.
10 Additional Study Procedures
10.1 Second Gasmet analyzer
A second Gasmet DX4040 analyzer was rented to reconfirm the key study results. This added 10 OnGuard/Tevadaptor replicates to the study that were tested with TMU surrogate. The testing with analyzer# 2 was identical to testing performed with analyzer# 1.
Late delivery of Gasmet analyzer# 2 and shortage in test products towards the end of the study limited the scale of testing with analyzer# 2.
Analyzer# 2 was also used to monitor BG levels to reconfirm BG levels of analyzer# 1.
10.2 5 Seconds “Measuring Time”
Additional 10 OnGuard/Tevadaptor replicates were tested with a different, a 5 seconds measuring time cycle. In this study, the analyzer’s “measuring time” (analysis time) is set to 1 second (the analyzer allows only 1, 5, 20 and 60 seconds measuring times). This is necessary as the vapor release during injection is just a few seconds long and dissolves very fast in the ambiance. Measuring with longer intervals misses the peak data points and analyzed concentration values appear lower. This additional testing session was performed to reconfirm that vapor escape from CSTDs is detected even with 5 seconds measuring time; and, reconfirm that 1 second setup is the right choice for this study.
1 1 Changes from Planned Study Procedures
The study protocol planed two injections of 50ml in the first and second stage into the same vial and same bag (mimicking 2g cyclophosphamide reconstitution with 100ml of diluent). This was changed at the beginning of the study after initial tests showed very high concentration values of vapor escape from CSTDs right on the first injection.
14 Conclusions
All four tested vented air-cleaning CSTDs and the syringes failed to contain vapor, and significant concentrations were released to the environment.
14.1 Tetramethylurea (TMU) surrogate:
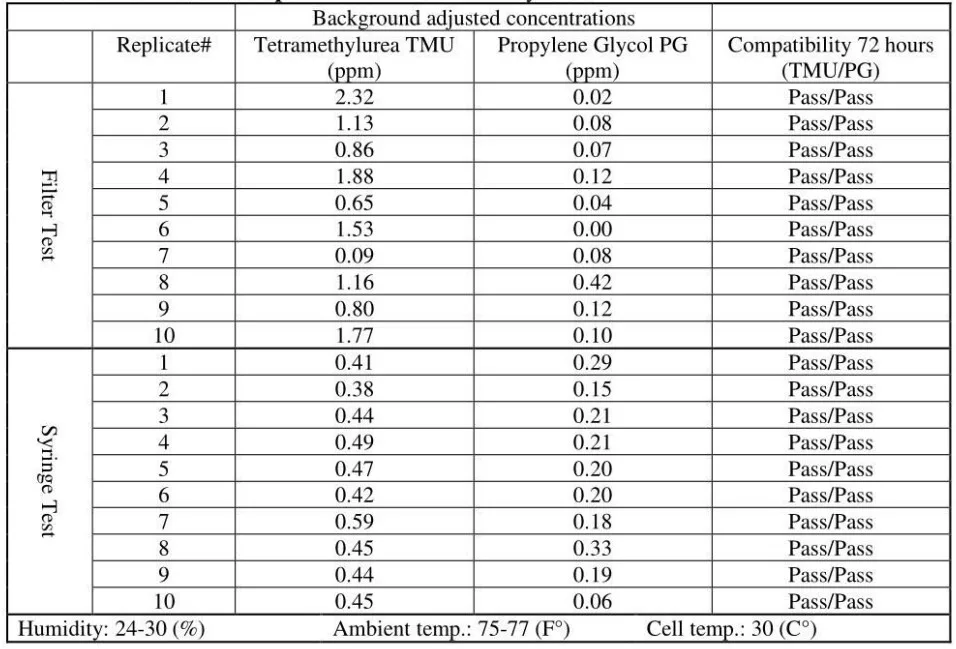
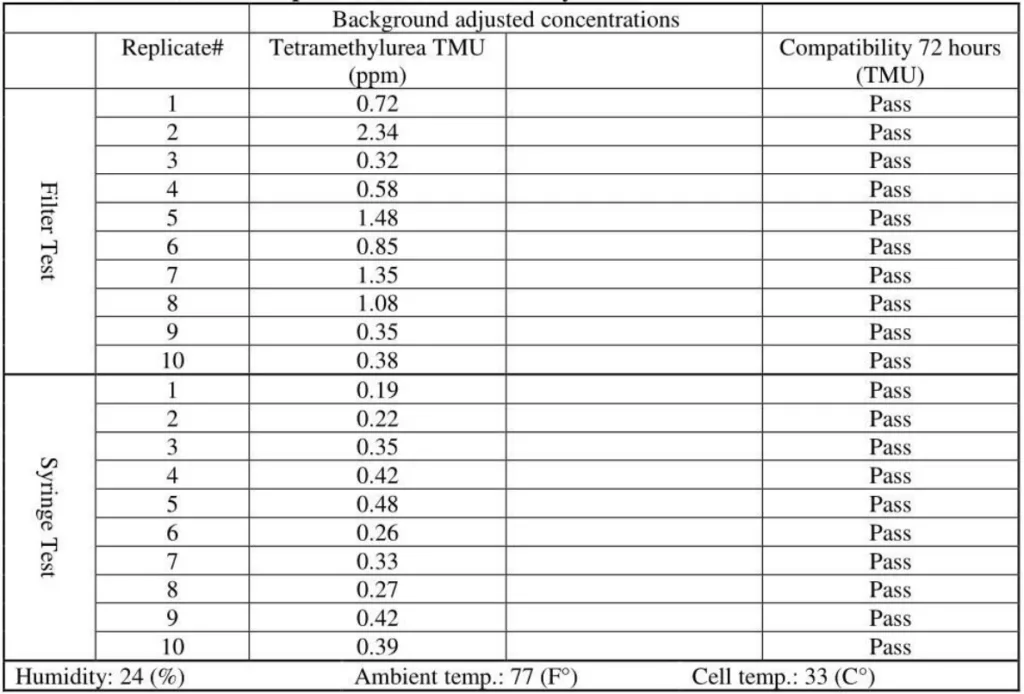
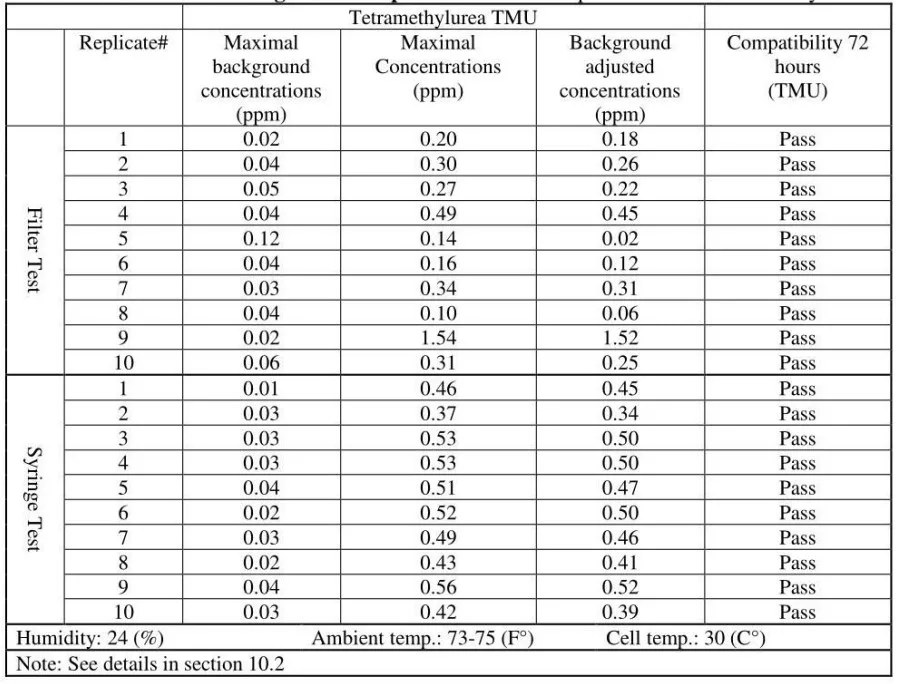
The released vapor concentrations mean values of OnGuard/Tevadaptor and Chemfort, which share the same filtration, were 1.22ppm, 0.95ppm, 1.4ppm and the maximum values were 2.32ppm, 2.34ppm and 2.45ppm (by groups as tested with both Gasmet analyzers# 1 and 2).
SmartSite mean values were 11.85ppm and the ChemoLock CSTD with 26.13ppm mean value released the highest vapor concentrations.
The mean vapor concentrations released from the open barrel of the syringes were relatively constant at 0.45ppm, 0.33ppm, 0.43ppm, 0.41ppm and 0.48ppm.
14.2 Propylene Glycol (PG) surrogate:
OnGuard/Tevadaptor and Chemfort, which share the same filtration, did not release PG vapor concentrations; their mean values were at 0.1Ippm and 0.02ppm, which are low close to background levels.
SmartSite and ChemoLock released vapor concentrations with mean values of 1.34ppm and 4.5ppm, respectively.
The mean vapor concentrations measured on the open barrel of the syringes were very low at 0.20ppm, 0.12ppm, 0.15ppm and 0.23ppm, very close to background levels.
14.3 Compatibility:
After 72 hours of storage at room temperature, all tested devices and vent filters maintained their functionality, mechanical integrity and didn’t leak.
14.4 Supplemental Testing
Although with slightly lower values, the second Gasmet analyzer# 2 reconfirmed the results of analyzer# 1 and reconfirmed the vapor escape from the tested CSTDs and syringes. Supplemental testing with larger analysis intervals (5 seconds) reconfirmed the correctness of this study setup.
14.5 Detailed Conclusions
- It can be concluded that TMU surrogate vapor was released in significant quantities from all CSTDs and syringes tested. TMU was compatible with the CSTDs and syringes under the study testing conditions. TMU is a suitable surrogate for testing CSTDs and syringes.
- It can also be concluded that PG surrogate vapor was not released from two of the CSTD brands tested and from syringes, although the same CSTDs and syringes did release vapor concentrations with the TMU surrogate. PG vapor was released from the other two CSTD brands but in significantly lower concentrations than with TMU. PG is not a suitable surrogate for testing CSTDs and syringes.
- It can be determined that vented air-cleaning CSTD can have varying vapor containment efficiencies with varying contaminants, and the same vented CSTD can contain vapor of one type of contaminants and absolutely fail to contain with others.
- It can also be determined that scientific evidence is established herewith for vapor release from regular open-barrel syringes as a route of exposure.
- It can be further determined that varying contaminants can cause varying levels of vapor contamination from open-barrel syringes, ranging from high contamination to none.
- It can be concluded that given the established fact of vapor release from regular open-barrel syringes as a route of exposure, the testing of syringes with 1PA alcohols alone is not suitable for performance protocol (NIOSH) because alcohol doesn’t adhere to syringe walls and alcohol is factually not detectable with any draft NIOSH protocol. Without the addition of a suitable surrogate for testing CSTDs and syringes, alcohol alone should not be used.
- It is appropriate to note that ineffective surrogates may lead users to make their selection of CSTDs that have no worker-protection performance.
Further research and testing are necessary. There are at least further nine surrogates on the NIOSH list of surrogates that should be evaluated. After years of research done by NIOSH, finding adequate surrogates that would cover all routes of exposure is the only acceptable solution.