Introduction
Some of tire drugs used in medical care (e.g., in cancer treatments) are extremely hazardous. Some of these compounds are volatile and might escape to the air in medical facilities. Long term exposine to such compounds, even at low concentrations, can lead to serious health risks to healthcare workers [1,2]. Therefore, it is important to reduce exposine by containing hazardous drug and its vapor releases using proper protective equipment. This can be achieved using specially designed CSTD systems. Some CSTDs that are venting air from drug vial headspace are equipped with air filtration technologies, that should prevent the escape of hazardous substances to the environment. Such systems are supposed to protect the operators from exposure.
Unfortunately, no performance standard for CSTDs has been published and it is not clear that the performance of all CSTD systems are adequate [3]. In this study, the vapor emission diuing CSTD-assisted transfer of water from a syringe to a vial containing drug surrogate was measured. Three filter CSTD systems were tested for preventing the leakage of vapors of three surrogates. Tire tests were carried out under conditions representing real usage of dings by healthcare workers, in an experimental setup that allows monitoring of the escaped vapors.
Experimental
Experimental
Three CSDT systems were compared: ChernoClave/Spiros (Type A, in the following), SmartSite/Texium (Type B) and OnGuard/Tevadaptor (type C). All units comprise two parts (adapters): one is fixed on tire syringe and the other is on the ding vial. Both Type A and Type B vial adapters contain a hydrophobic micronic filter; Type C vial adapters use a double-filter technology with a combination of micronic filter and an active carbon filter. Liquid can then be transferred from, or to the syringe, and the air pressure in the vial is equalized by air moving in or out of the system to environment through the filtered port.
Chemicals
The CSTDs were tested with three surrogates for hazardous dings from the list of nine agents proposed by NIOSH [4]: Tetramethylurea, Alfa Aesar, Thermo Fisher Scientific (United Kingdom), Tefraethylurea, Tokyo Chemical Industry Co. LTD (Japan), Propylene glycol (1,2-Propanediol), Chen Shmuel Chemicals LTD (Israel). All reagents were used as received. Ultrapure water (18.2 MfUcm. Direct-Q* 3) was used in all the experimental procedures.
Analyzer
The air samples were analyzed using the Gasmet DX4040 FTIR analyzer which is also used by NIOSH [5], The calibration earner gas used was nitrogen, 99.999% purity. The volume of its measurement chamber is 400 ml and the effective optical pass is 9.8 m. The analyzer was properly calibrated (by manufacturer) prior to the experiments and retested in our lab, using a standard mixture of gases.
The spectra of the tested surrogates and the relevant spectral range used for their analysis, are shown in Fig. 2.
Setup
A vial connected to a syringe through CSTD, was positioned at a 45-degree angle, as depicted in Fig. 1. It was placed between a glass funnel and a Buchner funnel, equipped with sintered glass filter. The air passed through the sinter glass filter, forming quasi-laminar flow. The air, together with the emitted contaminants were partially collected by the upper glass funnel and conducted to the analyzer via teflon tube. The air was circulated along this pass during the analyzer’s measurement time.
The following points were taken into consideration in the design of the experimental setup:
(a) The testing system is an opened one. Using a large chamber closed system, that mimics a room, would dilute the released chemicals below the instrumental detection limit. On the other hand, using a small volume chamber, would not properly represent real conditions, due to development of pressure differentials and due to large surface-to-volume ratio (chamber walls adsorb molecules). The chosen configuration avoids these dr awbacks since it is external to CSTD and doesn’t interfere with any known aspect of CSTD performance, but at a price: some molecules escaping the CSTD system may not reach the analyzer. This is not very important, since our task is to compare the performance of various CSTD systems and not to measure the absolute amount of chemicals released.
(b) Tire commonly used by healthcare workers inclined configuration (45-degree) was selected after testing many different angles and proper optimization. Horizontal air flow, migirt cause losing too much of the emitted molecules, which are either heavier or lighter than air. Vertical air flow is physically not possible and is not in use.
Table 1. CSTD containment performance data.
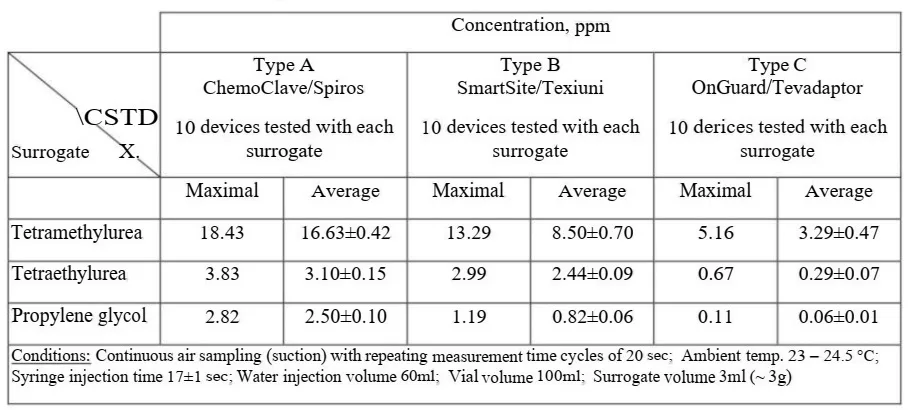
Fig. 1. The experimental setup, including placement of the syringe, vial with the test surrogate, CSTD system and the gas flow (black arrows)
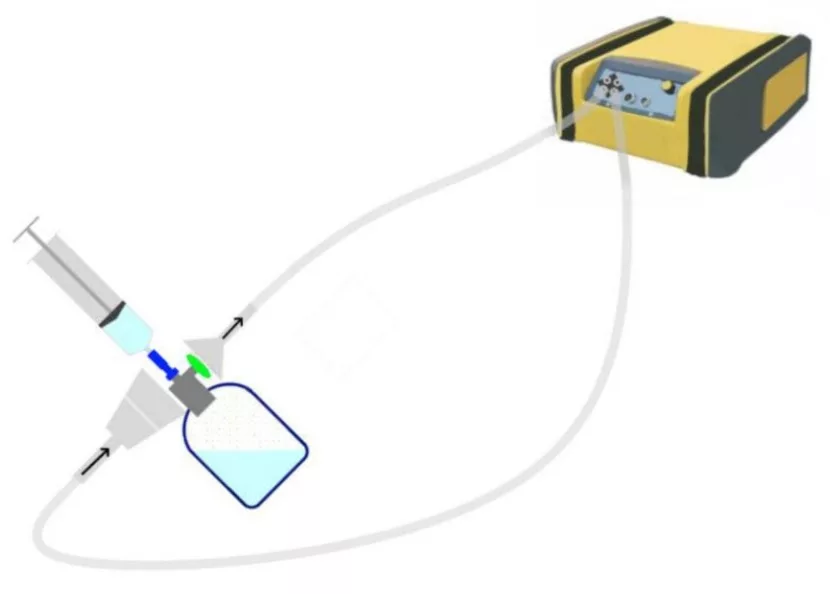
Fig. 2 The spectra of the tested substances. The wavelengths used for analysis are indicated.
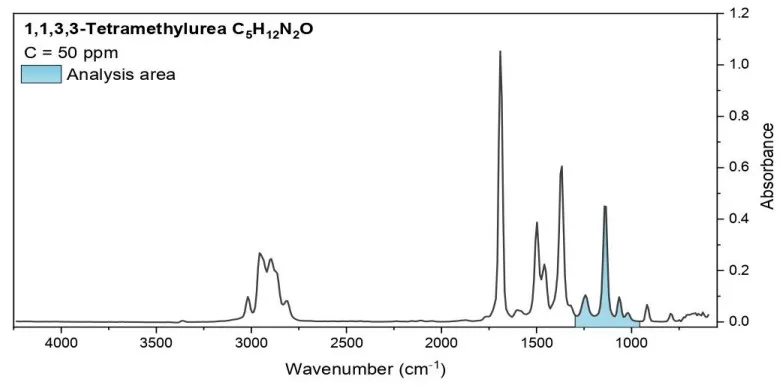
Analysis area: 958-1300 cm-1
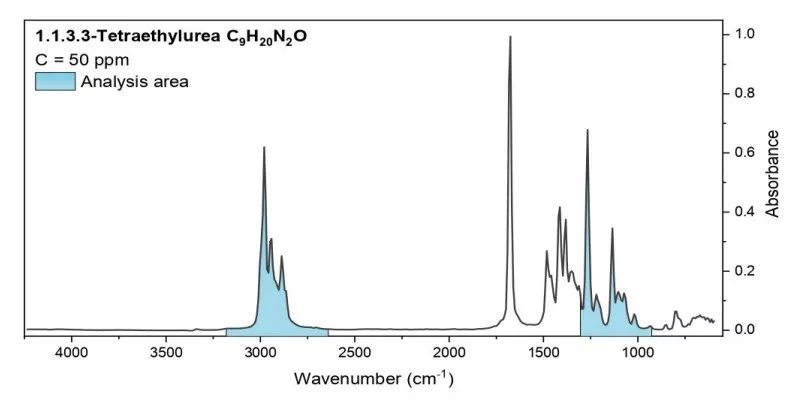
Analysis area: 925-1304 cm-1, 2640-3180 cm-1
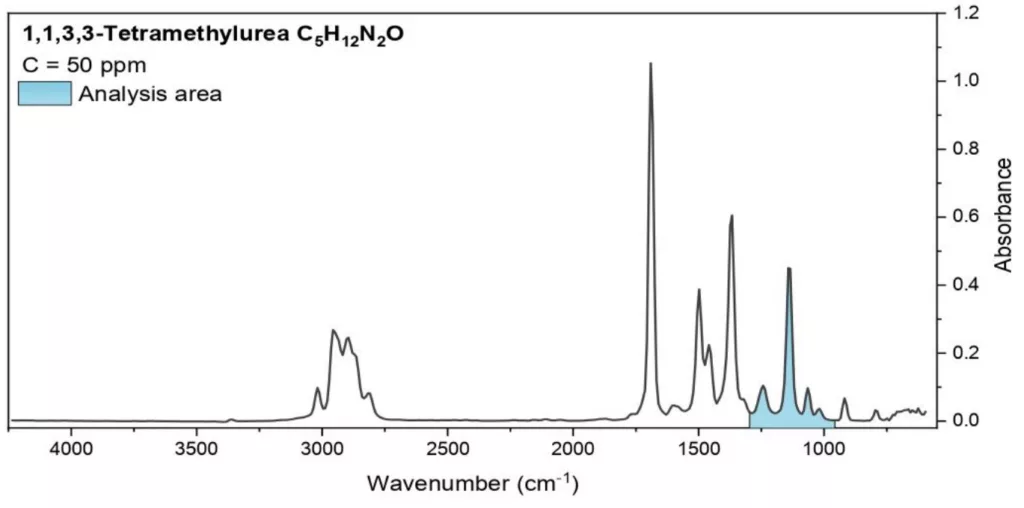
Analysis area: 895-1300 cm1
Procedures
Tire task of the procedure was to reproduce normal operation of tire CSTD system, as routinely done in hospitals. First, 100 ml glass vial containing 3 ml of a surrogate was prepared. To comply with real powder drugs that have drug coated inner walls, and in order to have standard conditions for the compar ison, the vial was rolled on its side, to achieve surface wetting, which does not depend on the dynamic conditions of the injection. However, care was taken not to allow surrogate contact with the vial’s rubber stopper. Then 60 ml of deionized water was injected to tire vial, using CSTD. The injection was carried out manually, and tire injection time was 16-18 s.
Concentrations were measured at sampling times of 20 s, (plus one second for writing and saving the data). At 20 s sampling time, water injection was started at the tenth second of the first measuring cycle. A new measurement began after the concentration reached zero. The highest data point of concentration was recorded for each surrogate.
All measurements were carried out at room temperature of 23 – 24.5 °C, while avoiding air turbulences.
Additional Study Procedures
Study development and optimization involved additional testing with significant variations of study parameters and surrogates. The additional testing included testing with sampling time of 1 s and 5 s in addition to 20 s. The concentrations recorded at 20 s sampling time are lower than those obtained at 5 s and tire highest values are recorded at 1 s sampling time. However, the better repeatability was obtained at 20 s sampling time. The reason is that longer sampling averages over high and low concentration values in the measurement chamber, while short sampling time can indicate high actual concentrations reached at certain time. Nevertheless, for the same reasons, the standard deviations are lower at longer sampling times. The results indicate that dining the injection, there might be moments of high release of the chemicals.
Results and discussion
The results for average maximum concentrations detected with Gasmet DX4040 FTIR analyzer for the three CSTDs and three drug surrogates, are shown in Table 1. The standard deviations of these averages, calculated from repeating die measurements 10 times, are also indicated.
Table 1. CSTD containment performance data.
The interpretation of these data is related to the experimental setup used and the measurement procedure. First, note that these concentrations are not room concentrations, but concentrations recorded in the analyzer’s measurement chamber. The concentrations start at zero, increase during the injection (due to emission of the compounds from the CSTD systems), and then decrease and return to zero. Quantification with this open system setup is limited to the highest value cited and absolute quantification is not possible. These values provide evidence for the escape of vapor from the tested CSTDs and can be used for comparing the performance of the examined systems.
In general, all three systems are leaking. However, there are differences between the tested systems. Type A and Type B CSTDs showed a relatively high level of leakage. Type C demonstr ated better filtration properties than the other two CSTDs.
There are also differences in the leakage of the 3 tested surrogates, up to a factor of 45. It means that tire CSTD’s performance strongly depends on the surrogate. Propylene Glycol was hardly or not detectable with Type C, but both other surrogates Tetramethyhuea and Tetraethylurea were clearly leaking from same Type C. Tire results indicate that perhaps Propylene Glycol is not a suitable testing agent for these safety devices. Tetramethylurea demonstr ated the highest concentration values and stability throughout the study with all three tested CSTDs.
Further research is needed for the CSTD performance protocol, with other NIOSH surrogates and testing methods.