Background
- The United Stites Pharmacopeia Chapter <797> standards state that single-dose vials (SDV) must be discarded 6 hours after the first vial access if accessed and kept in ISO class 5 air conditions, otherwise the vial should be discarded after 1 hour
- The purpose of this standard is to decrease the potential for bacterial contamination of medications. but this mandate leads to the waste of high-cost, chemically stable drugs
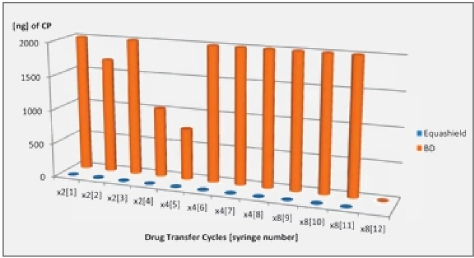
- The Equashield® closed-system transfer device (CSTD) was shown to prevent microbial contamination of preservative-free SDV for 9 days after being accessed 10 times over a 7 day period? and consequently the FDA approved extending the beyond-use date (BUD) of SDV to 7 days through the use of the Equashield® CSTD in May 2014’
- The Mount Sinai Hospital (MSH) has been using the Equashield® CSTD for the preparation and administration of hazardous drugs since 2011, and recently implemented BUD of chemo.biotherapy SDV in concordance with the recent FDA approval
- Our one month cost analysis study in 2013 estimated a potential cost savings of more than $20,000 per month by extending the BUD of SDV chemo/biotherapy to 7 days at our institution
Objectives
To assess the cost savings of extending the BUD of SDV of chemo/biotherapeutic agents through the use of the Equashieldt® CTSD
- Primary objectives
- To assess actual chemobiotherapy wastage: cost of chemobiotherapy discarded after implementing BUD of SDV
- To assess potential chemobiotherapy wastage: cost of chemobiotherapy that would have been discarded if BUD of SDV was not implemented
- Secondary objectives
- Total number parenteral chemobiotherapy preparations compounded
- Estimated cost of Equashieldt products used
- The combined cost of wasted chemobiotherapy and Equashiel® products
Methods
- A prospective economic analysis of all discarded liquid SDV of chemo/biotherapeubc agents from October 1st to October 30th 2014 (30-day study period) was performed at the MSH
- Wasted amount of the 28 eligible chemobiotherapeutic agents for the study (table 1) were documented on a daily basis
- The potential wastage of medications that would have been discarded if the vials were not reused for 7 days was also recorded
- 340B price was used for ambulatory use and non-340B price was used for inpatient use for this cost savings analysis
Discussion
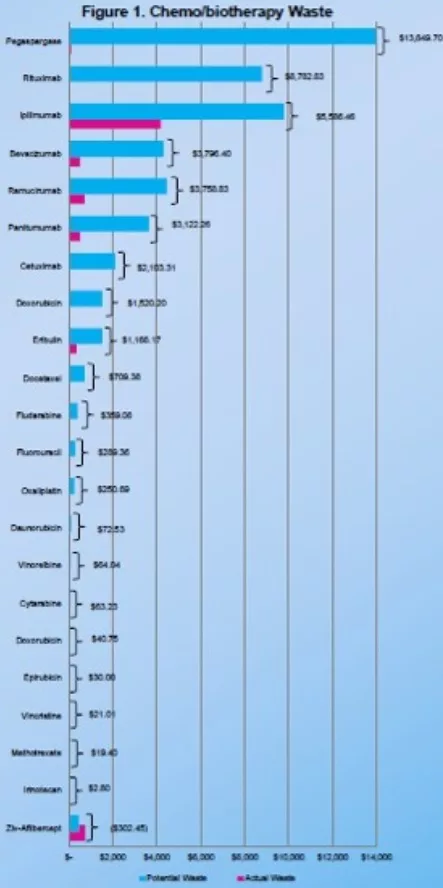
- Top six medications that represented the most cost savings were pegaspargase, rituximab, ipdimumab, bevacizumab, ramucirumab, and panitumumab
- Implementation of BUD of chemoibiotherapy SDV using Equashield® CSTD allowed for a significant cost savings of $44 192 during the one month study period, translating to an estimated cost savings of approximately $530,000 annually
- While implementing Equashield® CSTD represented an increase in annual expenditures of about $235,000, the resulting net cost savings by implementing BUD using this device ($530,000.’year) not only offset the cost of CTSD, but also resulted in a significant cost savings to our institution
Recommendation
- Cost analysis using a longer study period (3-6 months) will represent more accurate estimated annual cost savings
Limitations
- Due to the short study penod (30 days), chemo/biotherapeutic agents used during this study penod may not represent those used throughout the year
- BUD of chemo/biotherapy was implemented before the study period, therefore, our study included partiaty used vials that were initially opened before the study period, leading to potential underestimation of our cost savings
Disclosures
Authors of this presentation have nothing to disclose concerning possible financial or personal relationships with commercial entities that may have a direct or indirect interest in the subject matter of this presentation
Tables 1-2
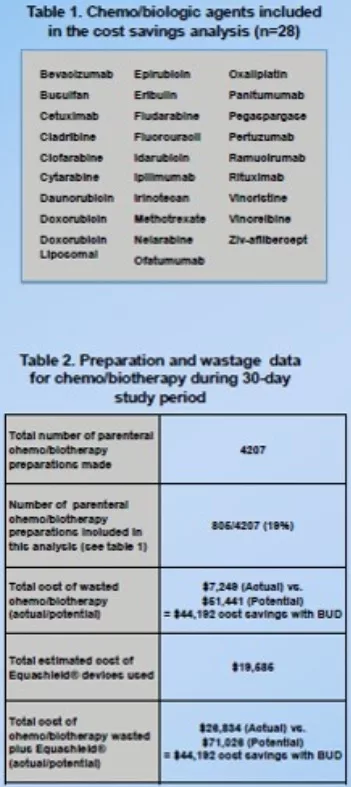
Figure 1