As the risks associated with occupational exposure to hazardous drugs become increasingly evident, growing awareness is given to the methods, procedures and means involved in the preparation and administration of such drugs. All aim to provide healthcare workers with maximum protection, by minimizing the contact of hazardous drugs with
their immediate environment. Various reputable
publications such as NIOSH Alert (2004)1 and ASHP Guidelines on Handling Hazardous Drugs (2006)2, highlight the effectiveness of implementing appropriate working practices and using adequate protective equipment. Both NIOSH1 and ISOPP7 have recommended the u se o f clo sed sy s tem transfer devices (CSTD), prohibiting the escape of contaminants into the environment, as a vital part
of any protective equipment. Similarly, the effectiveness of CSTD has been well documented in numerous studies.
The effcacy and necessity of CSTD is unanimous and well established among relevant safety organizations. The compliance of available equipment with the deffnition of a CSTD as “a closed system drug transfer device [that] mechanically prohibits the transfer of environmental contaminants into the system and the escape of hazardous drugs and vapor concentrations into the environment ” is still
ambiguous and contradictory.
Under normal working conditions, drugs tend to evaporate in gaseous form into the preparation site ambient air. Their condensation may contaminate: work surfaces, biological safety cabinets (BSC), preparation rooms, equipment, gloves and gowns, as well as the prepared IV bags and syringes in the BSC, that are ready to be sent to the administration area (Kifmeyer, Kube, Opiolka, Schmidt, Schöppe, Diplom Volkswirt, and Sessink, 20024 ; Vandenbroucke and Robays, 20015).
Furthermore, inhalation of toxic vapor evaporating during preparation is suspected to be one of the main routes of exposure.
Studies by Thekla K.Kiffmeyer and Kube et al.4, Vandenbroucke and Robays5, and Connor, Shultsb and
Fraserc6 , demonstrate the behavior of hazardous drug vapors and the ineffciency of fflters in protecting against exposure to such vapor. These findings must be considered when designing safety measures.
Furthermore, although CSTD is only one essential aspect in an overall set of measures that must be applied, it is clear that setting definite criteria for CSTD is required in order to ensure that only safe, completely leak-proof and airtight devices capable of providing genuine protection are accepted and
used as a CSTD. Using a genuine CSTD can signiffcantly improve the safety of healthcare workers.
In the current test, a replication of a test performed by Jorgenson & Cam Au et al.3 at the University of Utah Health Care, air injected by a syringe sweeps the Titanium tetrachloride vapor from the vial. In the case of filter venting based systems, the swept vapor passes unhindered through the filter into the environment. Titanium rapidly reacts with atmospheric moisture: TiCl4 + 2H2 O > TiO 2 + 4HCl. The hydrogen chloride absorbs water to form tiny droplets of
hydrochloric acid, which may absorb more water to produce large droplets that effciently scatter light. In addition, the intensely white titanium dioxide is also an effcient agent for scattering light. The smoke generating at the filter’s exterior is clearly visible, demonstrating the vapor’s behavior. Another portion of smoke is generated inside the vial, through the reaction with the moisture in the air coming from the
syringe. Some smoke particles that are too large to pass through the filter remain in the vial.
Objective
Thee purpose of this study was to evaluate the vapor containment effciency of several commercially available devices, mainly by grouping them into two main categories: filter venting based systems vs. pressure equalization based systems, in order to evaluate the airtight sealing properties of each category and to determine which devices can prevent the escape of vapor during the preparation and administration of
hazardous drugs.
Method
The following drug transfer devices were tested for vapor containment effectiveness, using vials with Titanium tetrachloride as a drug substitute. Titanium tetrachloride acts as a vapor simulator, generating clearly visible smoke when reacting with atmospheric moisture. Each device was observed during its operation for any release of Titanium into the environment. 27ml and 7ml glass vials were filled with 3ml and 1.5ml Titanium tetrachloride TiCl4 (purum ≥99.0%), respectively.
A 20mm crimper was used to seal the vials with vial stoppers and 20mm aluminum crimp seals with flip-off caps. Tge filling and sealing procedures were performed in an ultra dry environment in an airtight glove box. The sealed vials were taken out of the glove box and the remaining procedure was conducted under ambient conditions. Each system was tested separately, beginning with assembly according to the manufacturer’s instructions, and attachment of a 20ml luer-lock Becton & Dickinson syringe, filled with 20ml of environmental air. Each system is equipped with a vial access adaptor, which was attached to the vial. The syringe with each system’s dedicated connector was connected to the vial adaptor and 20ml of air was injected manually during 5 seconds at a constant speed. Each system was tested with 27ml and 7ml vials.
Results
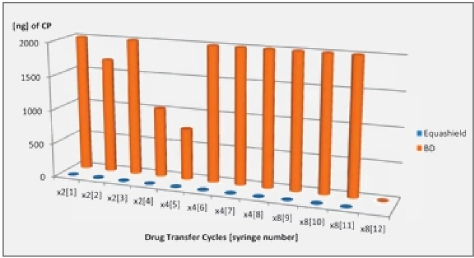
Only the closed systems with full pressure equalization, i.e. Equashield™ and Phaseal® prevented the release of Titanium tetrachloride into the environment and complied with the NIOSH deffnition for a Closed System Drug Transfer Device (see Figures 1 and 2).
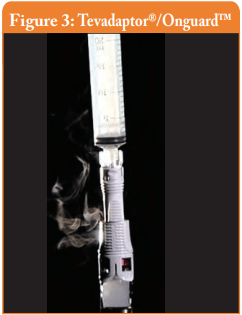
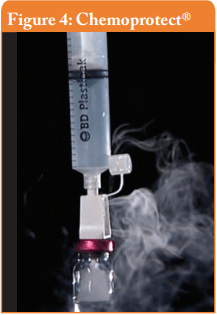
Filter venting systems, i.e. Tevadaptor®/Onguard™ and Chemoprotect® spike, were consistently releasing similar and clearly visible smoke during all tests, with both vial sizes (see Figures 3 and 4). There were negligible differences in Titanium release severity between the vial sizes. The filter of Chemoprotect® spike is clearly visible on one side of the device and the release of Titanium was deff- nitely observed only through the filter. The two filter layers (particle filter and activated charcoal) of Tevadaptor®/Onguard™ are invisible from the outside and are covered by an easily removable housing, which does not affect the device performance. The Titanium test was performed on an additional 5 exposed devices, confirming that the Titanium release occurs only through the filters.
After testing, filters of Tevadaptor®/Onguard™ and Chemoprotect®, were carefully inspected by an x8 magnifying glass, for any evidence of leaks and damages which could have contributed to the escape of the Titanium. As in previous tests performed by Jorgenson & Cam Au et al.3 at the University of Utah, and by the SP National Testing and Research Institute in Borås, Sweden3, where filters were gold coated and viewed under electron microscopy, no evidence of such damage was found.