Introduction
Hazards associated with handling of chemotherapy drugs are well documented1-11. Ensuring healthcare worker safety should be a priority and organizations are wise to invest significant time in development of a comprehensive HD safety programs.
Guidelines provided by NIOSH Alert1, ASHP recommendations2 and Proposed USP<800>3 offer a large number of steps needed to safely compound hazardous drugs. As healthcare shifts to a model where improved efficiencies and reduced labor and supply costs are critical, it is important that number of steps and time to compound a dose be considered when a closed system drug transfer device is being chosen.
Objectives
The key objective of this study is to clarify the misperceptions surrounding CSTDs. Over the last 15 years, CSTDs have evolved in technology and offer various mechanism for containing vapor and protecting healthcare workers. This study aims at assessing various technologies for Hazardous Drug Compounding by performing a Time-Motion assessment. The study looks at both total steps and total time to compound a simulated dose of chemotherapy using the following ONB approved CSTDs:
- BD PhaSeal
- ICU ChemoLock
- Equashield
The study will also qualitatively assess key attributes that lead to increase in efficiency.
Methods
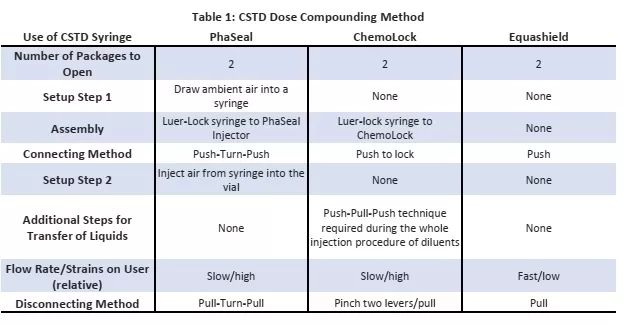
Each of the 3 tested CSTDs were assessed across the same set of compounding protocol to prepare an IV Piggyback dose from a liquid dose vial. Table 1 below outline the high level method and Figure 2 shows the CSTD Setup Structure.
Results
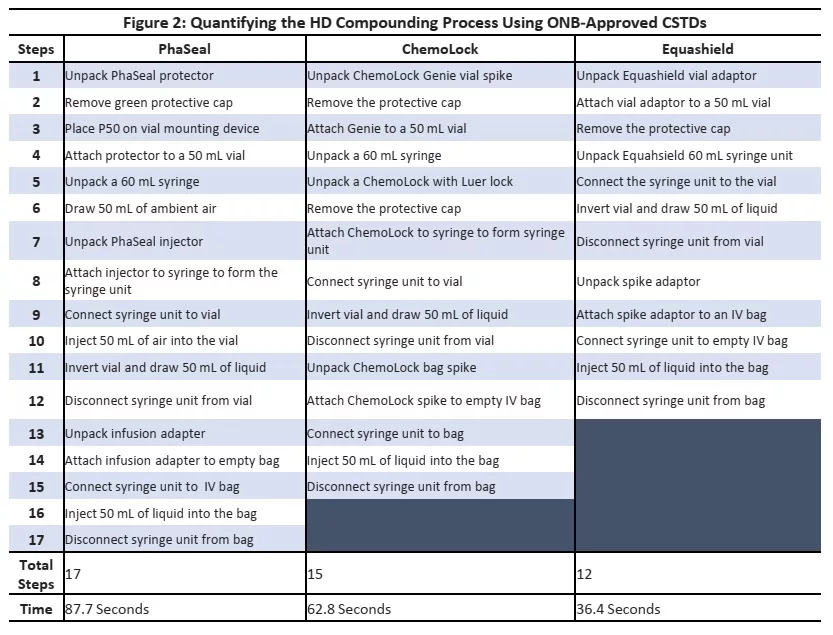
Figure 2 below summarizes outlines the process steps and time needed to compound a dose of chemotherapy using various CSTDs.
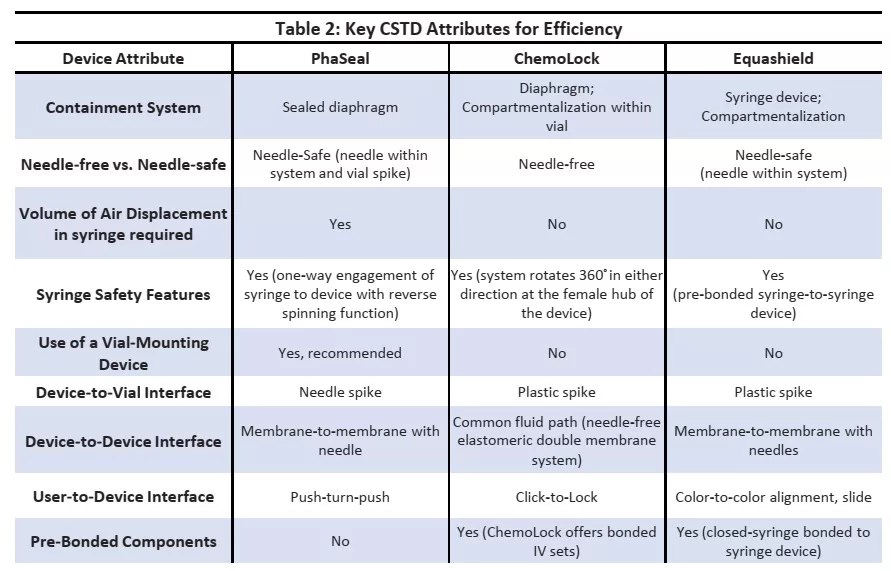
It must be noted that process steps and compounding mechanisms were different across CSTDs tested. The time required to compound a dose correlated with the number of steps needed to complete a preparation. Table 2 below outlines the key CSTD attributes that contribute the increase in efficiency of one system relative to another.
The process step that contributed to most time required to compound a dose with PhaSeal was the air displacement step. Due to product design, it was required to introduce premeasured air into the syringe prior to syringe adaptor connection.
Similarly, for ICU the largest time consuming step was the Push- Pull-Push technique for injection procedure of diluents. It must also be noted that both PhaSeal and ChemoLock required use of standard syringe, while Equashield offered closed-syringe bonded to syringe devices. Equashield also required the least number of steps and overall time to compound a dose given design attributes that lead to optimal efficiency of the product for use when compounding hazardous drugs within a pharmacy.
Conclusion
CSTDs are proven to reduce exposure to HDs during the drug compounding and administration processes. Contrary to common belief, when staff is properly trained and are experienced CSTD users, the time required to compound CSPs using CSTDs does not differ significantly from the time
it takes to compound with a needle and syringe. Although this analysis shows variability in the time required for compounding using the three CSTDs evaluated, all the CSTDs increase safety
without adding an untenable amount of time to work to the process.
Understanding the impact of CSTDs on pharmacy compounding workflow and output is critical. In addition to safety, CSTDs should facilitate efficiency. Critically reviewing the steps for using each CSTD and summarizing the differences in mechanical manipulation can help assess the time required to compound CSPs using CSTDs.
Once the number of steps required and the time for the compounding process are determined, multiplying these metrics by number of doses compounded daily, weekly and annually will allow managers to quantify the time required for compounding over a given time period. In this way, managers can determine workload requirements and monitor the need for additional personnel or the reduction of hours based on changing compounding volume.

Key take away from the study can be summarized below:
- PhaSeal required the most steps to compound a dose (17 steps) while Equashield required the least (12 steps).
- Similarly PhaSeal required over twice the time to compound a dose compared to Equashield
- ChemoLock performed in the middle with 15 steps and 62.8 seconds to compound a dose
As hospital budgets are trimmed and focus on cost cutting increases, it is important to select a closed system that is both safe and efficient for compounding Hazardous Drugs.