Introduction
Hazards associated with handling of chemotherapy drugs are well documented [1-3]. Ensuring healthcare worker safety should be a priority and organizations are wise to invest significant time in development of a comprehensive HD safety programs. Guidelines provided by NIOSH Alert[1], ASHP recommendations[2] and Proposed USP<800>[3] offer a list of process steps needed to safely compound hazardous drugs. As the new NIOSH proposed CSTD test protocol comes into play, it is crucial to test all aspects of closed systems, exposure containment, fully airtight design and equally importantly a dry, leak free design. This is important as facilities are quickly able to perform bench top testing to assess ‘closeness’ of devices
Objectives
Over the last 15 years, CSTDs have evolved in technology and offer various mechanism for containing liquid and protecting healthcare workers. Some systems perform better than others and is a pure correlation of product design and materials chosen for prevention of leaks and spills. The key
objective of this study is to assess how one Closed System Transfer Device, a newest addition in the market, compares with its claims to be leak-free and dry for up to 10 connections or membrane activations. This study looks only one Closed System Transfer Device; the second generation Equashield CSTD was assessed against a predefined and
controlled protocol in a hospital facility to validate or invalidate
manufacturer’s claims.
Tests performed with 3 different PH liquid and was qualitative in nature.
Materials
To assess whether the Closed System is dry, it will be tested against several solutions to mimic various drugs’pH levels seen in chemotherapy compounding on a routinebasis.To perform this test the followingmaterialswere used:
- 10 Vials with pH 4 liquid solution
- 10 Vials with pH 7 liquid solution
- 10 Vials with pH 10 liquid solution
- 30 Equashield VA-20/2 Vial Adaptors
- 30 Equashield SU-EZ60/2 Syringe Units
- Litmus Paper
- Data Collection Sheets per protocol
Prior to start of the test, 10 vials each of varying pH solutions were prepared for assessment in lieu of actual drugs for a total of 30 vials.
Figure 1: Sample Preparation Process

pH Test
Methods
All necessary supplies were gathered for testing and following process steps were performed:
- A vial of pH 4 vial was retrieved
- Corresponding data collection sheet was retrieved
- Vial was fitted with VA-20/2 vial adaptor as per manufacturer’s instructions per use
- A SU-EZ60/2 syringe unit was retrieved and connected to the vial with vial adaptor
- A small volume of fluid was transferred from the vial into the syringe unit
- Syringe unit was disconnected from the vial with vial adaptor
- With a litmus paper both membranes (vial adaptor membrane and syringe unit membrane) were assessed.
- If the litmus paper changed color, it was marked as ‘x’ on the data collection sheet (denoting system failure). If the litmus paper did not change color, it was marked as ‘y’ on the data collection sheet (denoting that system passed the test).
- After the 1st vial connection and disconnection, the same syringe and vial assembly were connected again, fluid was transferred, disconnected and membrane tested for wetness/color change to denote 2nd connection or membrane activation
- This action was performed up to 10 connection times per vial and pH solution
Data collection sheets were effectively populated for all test samples for a total of 3 buffer solutions, 10 vials per solution and 10 activations per vial totaling 300 data points.
Results
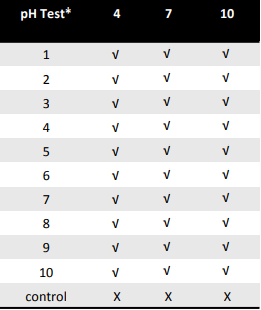
After performing the dry connection effectiveness test for 300 samples, 0 failures were documented. None of the samples tested across all 3 pH levels created leaks or wet membranes. Furthermore, it should be noted that the controls were positive, confirming the integrity of the test solution. Figure 3 outlines the summary results of the test.
* Each test included 10 manipulations
√ denotes no residuals detected — X denotes residue was detected
Conclusion
Key take away from the study can be summarized below:
- Commonly found pH levels were tested in this protocol to
assess its ability to remain dry - 300 measurements were generated by this study protocol with no residues found on the surface in any sample
- Equashield was put to the test for its claim of being able to maintain a dry connection for up to 10 activations and passed the test
Equashield was leak free and dry and meets the NIOSH definition of a closed system transfer device with respect to its ability to maintain dry connections validating vendor’s claims.